The role of platelet-specific autoantibodies in clinical features and prognosis evaluation of patients with immune thrombocytopenia
-
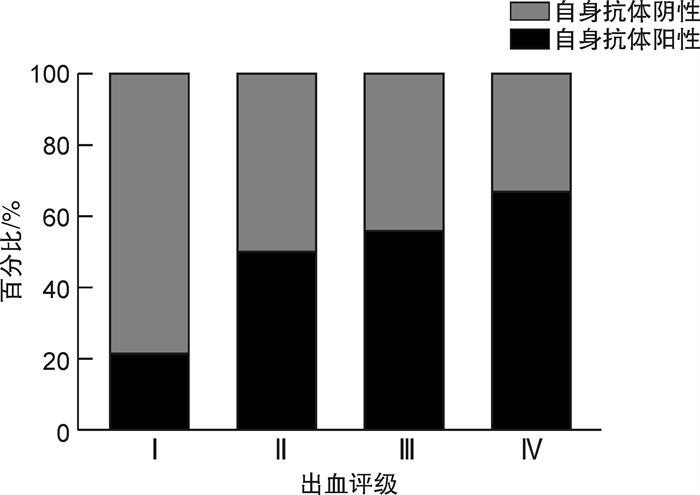
摘要: 目的 评估血小板特异性自身抗体在免疫性血小板减少症(ITP)患者临床表现、一线治疗的疗效反应和预后评估中的作用。方法 对2019年12月—2021年12月在我院就诊的249例ITP患者进行回顾性研究,其中男98例,女151例,中位年龄50(30~60)岁,入院时中位血小板计数为13×109/L(3×109/L,28×109/L),利用流式微球技术进行血小板特异性自身抗体的检测。结果 249例ITP患者中新诊断期99例,持续期53例,慢性期97例。抗体检测结果显示,23例患者具有抗血小板表面糖蛋白(GP)Ⅰbα自身抗体,22例患者具有抗GPⅡb/Ⅲa自身抗体,50例患者同时检测到上述两种抗体,154例患者未检测到上述两种抗体。血小板特异性自身抗体阳性患者的出血评分4(4,5)明显高于无自身抗体的患者3(0,5)(P< 0.05)。线性关联χ2检验表明,随着出血等级的增加,抗体阳性率呈线性增加(χ2=20.097,P< 0.001)。在99例新诊断的ITP患者中,自身抗体阳性患者对标准一线治疗的反应率(42.1%,16/38)显著低于无自身抗体的患者(72.1%,44/61)(P< 0.05)。二元logistic回归分析显示,血小板特异性自身抗体的存在对新诊断ITP患者一线治疗的疗效有显著影响(P=0.006,OR=0.277,95%CI0.110~0.695)。结论 血小板特异性自身抗体阳性的ITP患者出血风险较高,对一线治疗的反应较差,更容易转变为慢性期,需要对具有血小板特异性自身抗体的ITP患者进行个体化治疗。
-
关键词:
- 免疫性血小板减少症 /
- 血小板特异性自身抗体 /
- GPⅠbα /
- GPⅡb/Ⅲa /
- 一线治疗
Abstract: Objective To evaluate the role of platelet-specific autoantibodies in clinical manifestations, efficacy of first-line treatment and prognosis of patients with immune thrombocytopenia(ITP).Methods A retrospective study was performed on 249 ITP patients who were treated in our hospital from December 2019 to December 2021. There were 98 males and 151 females, the median age was 50(30-60) years, and the median platelet count at admission was 13×109/L(3×109/L, 28×109/L). Platelet-specific autoantibodies were detected using cytometric bead array(CBA).Results Among the 249 ITP patients, 99 cases were newly diagnosed, 53 cases were in the persistent phase, and 97 cases were in the chronic phase. CBA results showed that 23 patients had anti-platelet surface glycoprotein GPⅠbα autoantibodies and 22 patients had anti-GPⅡb/Ⅲa autoantibodies. Both antibodies were detected in 50 patients and none were detected in 154 patients. Patients with autoantibody-positive had significantly higher bleeding scores 4(4, 5) than that of 3(0, 5) in patients without autoantibodies(P< 0.05). The linear association chi-square test showed that with the increase of bleeding grade, the antibody positive rate increased linearly(χ2=20.097,P< 0.001). Among 99 newly diagnosed ITP patients, the response rate of autoantibody-positive patients to standard first-line therapy(42.1%, 16/38) was significantly lower than that of patients without autoantibodies(72.1%, 44/61)(P< 0.05). Binary logistic regression analysis showed that the presence of platelet-specific autoantibodies had a significant effect on the efficacy of first-line treatment in newly diagnosed ITP patients(P=0.006,OR=0.277, 95%CI0.110-0.695).Conclusion ITP patients with platelet-specific autoantibodies have a higher risk of bleeding and a poor response to first-line therapy. Individualized treatment is needed for patients with autoantibodies.-
Key words:
- immune thrombocytopenia /
- platelet-specific autoantibodies /
- GPⅠbα /
- GPⅡb/Ⅲa /
- first-line therapy
-

-
表 1 入组患者临床特征
临床特征 GPⅠbα(+) GPⅠbα(-) GPⅡbⅢa(+) GPⅡbⅢa(-) GPⅡbⅢa(+) GPⅡbⅢa(-) 例数 249 50 23 22 154 男∶女/例 98∶151 12∶38 12∶11 10∶12 64∶90 年龄/岁 50(30,60) 44(28,56) 52(28,60) 50(31,57) 50(31,61) 入院时血小板计数/(×109·L-1) 13(3,28) 11(3,29) 11(6,18) 15(2,25) 13(3,29) 出血评分 4(2,5) 4(4,5) 4(3,5) 5(4,5) 3(0,5) 分期/例 新诊断 99 19 12 7 61 持续期 53 7 5 3 38 慢性期 97 24 6 12 55 表 2 影响患者一线治疗反应性的二元logistic回归分析
因素 P Exp(B) 95%CI for Exp(B) 下限 上限 性别 0.107 2.090 0.853 5.117 年龄 0.353 1.011 0.988 1.035 血小板特异性自身抗体 0.006 0.277 0.110 0.695 出血评分 0.773 0.957 0.711 1.289 入院时血小板计数 0.750 0.997 0.977 1.017 常数 0.620 0.606 -
[1] Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia[J]. Blood, 2010, 115(2): 168-186. doi: 10.1182/blood-2009-06-225565
[2] McMillan R, Tani P, Millard F, et al. Platelet-associated and plasma anti-glycoprotein autoantibodies in chronic ITP[J]. Blood, 1987, 70(4): 1040-1045. doi: 10.1182/blood.V70.4.1040.1040
[3] van Leeuwen EF, van der Ven JT, Engelfriet CP, et al. Specificity of autoantibodies in autoimmune thrombocytopenia[J]. Blood, 1982, 59(1): 23-26. doi: 10.1182/blood.V59.1.23.23
[4] Chaturvedi S, Arnold DM, McCrae KR. Splenectomy for immune thrombocytopenia: down but not out[J]. Blood, 2018, 131(11): 1172-1182. doi: 10.1182/blood-2017-09-742353
[5] Cines DB, Blanchette VS. Immune thrombocytopenic purpura[J]. N Engl J Med, 2002, 346(13): 995-1008. doi: 10.1056/NEJMra010501
[6] Jansen AJ, Josefsson EC, Rumjantseva V, et al. Desialylation accelerates platelet clearance after refrigeration and initiates GPIbalpha metalloproteinase-mediated cleavage in mice[J]. Blood, 2012, 119(5): 1263-1273. doi: 10.1182/blood-2011-05-355628
[7] Li J, van der Wal DE, Zhu G, et al. Desialylation is a mechanism of Fc-independent platelet clearance and a therapeutic target in immune thrombocytopenia[J]. Nat Commun, 2015, 6: 7737. doi: 10.1038/ncomms8737
[8] Grozovsky R, Begonja AJ, Liu K, et al. The Ashwell-Morell receptor regulates hepatic thrombopoietin production via JAK2-STAT3 signaling[J]. Nat Med, 2015, 21(1): 47-54. doi: 10.1038/nm.3770
[9] Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group[J]. Blood, 2009, 113(11): 2386-2393. doi: 10.1182/blood-2008-07-162503
[10] George JN, Woolf SH, Raskob GE, et al. Idiopathic thrombocytopenic purpura: a practice guideline developed by explicit methods for the American Society of Hematology[J]. Blood, 1996, 88(1): 3-40. doi: 10.1182/blood.V88.1.3.3
[11] Chen Y, Xie Y, Ruan M, et al. The Levels of T Lymphocyte Subsets in Immune Thrombocytopenia Associated with Anti-GPⅡb/Ⅲa- and/or Anti-GPIbα-Mediated Responses Are Differentially Sensitive to Dexamethasone[J]. Acta Haematol, 2018, 140(1): 60-66. doi: 10.1159/000491977
[12] Hauch TW, Rosse WF. Platelet-bound complement(C3) in immune thrombocytopenia[J]. Blood, 1977, 50(6): 1129-1136. doi: 10.1182/blood.V50.6.1129.1129
[13] Tsubakio T, Tani P, Curd JG, et al. Complement activation in vitro by antiplatelet antibodies in chronic immune thrombocytopenic purpura[J]. Br J Haematol, 1986, 63(2): 293-300.
[14] Takahashi R, Sekine N, Nakatake T. Influence of monoclonal antiplatelet glycoprotein antibodies on in vitro human megakaryocyte colony formation and proplatelet formation[J]. Blood, 1999, 93(6): 1951-1958. doi: 10.1182/blood.V93.6.1951.406a33_1951_1958
[15] Tao L, Zeng Q, Li J, et al. Platelet desialylation correlates with efficacy of first-line therapies for immune thrombocytopenia[J]. J Hematol Oncol, 2017, 10(1): 46. doi: 10.1186/s13045-017-0413-3
[16] Liu XG, Ma SH, Sun JZ, et al. High-dose dexamethasone shifts the balance of stimulatory and inhibitory Fcgamma receptors on monocytes in patients with primary immune thrombocytopenia[J]. Blood, 2011, 117(6): 2061-2069. doi: 10.1182/blood-2010-07-295477
[17] Cheng Y, Wong RS, Soo YO, et al. Initial treatment of immune thrombocytopenic purpura with high-dose dexamethasone[J]. N Engl J Med, 2003, 349(9): 831-836. doi: 10.1056/NEJMoa030254
[18] Frederiksen H, Ghanima W. Response of first line treatment with corticosteroids in a population-based cohort of adults with primary immune thrombocytopenia[J]. Eur J Intern Med, 2017, 37: e23-e25. doi: 10.1016/j.ejim.2016.09.001
[19] Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin[J]. Annu Rev Immunol, 2008, 26: 513-533. doi: 10.1146/annurev.immunol.26.021607.090232
[20] Segal JB, Powe NR. Prevalence of immune thrombocytopenia: analyses of administrative data[J]. J Thromb Haemost, 2006, 4(11): 2377-2383. doi: 10.1111/j.1538-7836.2006.02147.x
[21] Mahévas M, Patin P, Huetz F, et al. B cell depletion in immune thrombocytopenia reveals splenic long-lived plasma cells[J]. J Clin Invest, 2013, 123(1): 432-442. doi: 10.1172/JCI65689
[22] Feng R, Liu X, Zhao Y, et al. GPⅡb/Ⅲa autoantibody predicts better rituximab response in ITP[J]. Br J Haematol, 2018, 182(2): 305-307. doi: 10.1111/bjh.14782
[23] Zeng Q, Zhu L, Tao L, et al. Relative efficacy of steroid therapy in immune thrombocytopenia mediated by anti-platelet GPⅡbⅢa versus GPIbalpha antibodies[J]. Am J Hematol, 2012, 87(2): 206-208. doi: 10.1002/ajh.22211
[24] Peng J, Ma SH, Liu J, et al. Association of autoantibody specificity and response to intravenous immunoglobulin G therapy in immune thrombocytopenia: a multicenter cohort study[J]. J Thromb Haemost, 2014, 12(4): 497-504. doi: 10.1111/jth.12524
[25] Frelinger AL 3rd, Grace RF, Gerrits AJ, et al. Platelet function tests, independent of platelet count, are associated with bleeding severity in ITP[J]. Blood, 2015, 126(7): 873-879. doi: 10.1182/blood-2015-02-628461
[26] Li J, Callum JL, Lin Y, et al. Severe platelet desialylation in a patient with glycoprotein Ⅰb/Ⅸ antibody-mediated immune thrombocytopenia and fatal pulmonary hemorrhage[J]. Haematologica, 2014, 99(4): e61-e63. doi: 10.3324/haematol.2013.102897
[27] 陈洋, 闫薛, 张婷, 等. 激素无效型免疫性血小板减少症患者T淋巴细胞亚群与血小板特异性自身抗体的相关性分析[J]. 临床血液学杂志, 2021, 34(11): 807-810. https://t.cnki.net/kcms/detail?v=znUxuWmAUtcVP4LNceAsXvQ8Gd7zLsfIfYId-SVnE1VhqR91yN2UO72Q4wtFuJSUNXPOde9vaq5CW4Cy5iOxH53KjNknjojjTDlCYr5LO4NCVvJrzKwXEqNWofr5cFnB&uniplatform=NZKPT
[28] Kapur R, Aslam R, Speck ER, et al. Thrombopoietin receptor agonist(TPO-RA)treatment raises platelet counts and reduces anti-platelet antibody levels in mice with immune thrombocytopenia(ITP)[J]. Platelets, 2020, 31(3): 399-402. doi: 10.1080/09537104.2019.1624709
[29] Swinkels M, Rijkers M, Voorberg J, et al. Emerging Concepts in Immune Thrombocytopenia[J]. Front Immunol, 2018, 9: 880. doi: 10.3389/fimmu.2018.00880
[30] Zheng SS, Ahmadi Z, Leung HHL, et al. Antiplatelet antibody predicts platelet desialylation and apoptosis in immune thrombocytopenia[J]. Haematologica, 2022 Feb 24. doi: 10.3324/haematol.2021.279751.Onlineaheadofprint.
-




 下载:
下载: