Effect of serological status of donor CMV and EBV on clinical prognosis in patients with allogeneic hematopoietic stem cell transplantation
-
摘要: 目的 研究供者CMV与EBV血清学状态对异基因造血干细胞移植(allo-HSCT)后患者的CMV与EBV激活、临床特征及预后的影响。方法 纳入2017年9月—2020年9月我院215例allo-HSCT患者及相应的供者,统计供、受者移植前CMV与EBV血清学状态,并持续监测移植后患者CMV和EBV DNA拷贝数至移植后至少6个月,采用χ2检验和Kaplan-Meier法及Cox回归模型分析CMV及EBV病毒血症的发生情况及临床随访资料。结果 移植后CMV D+/R+组和D-/R+组、D+/R-组和D-/R-组患者的CMV病毒血症的中位发生时间、CMV最高病毒拷贝数的中位数、CMV与EBV共激活率、CMV激活率、CMV病毒持续阳性率、移植后急性移植物抗宿主病(aGVHD)、慢性移植物抗宿主病(cGVHD)、移植后淋巴增殖性疾病(PTLD)、CMV肺炎发生率、2年总生存(OS)率、非复发死亡率(NRM)比较,差异均无统计学意义。移植后CMV D-/R+组CMV病毒反复激活率、出血性膀胱炎发生率明显高于D+/R+组(P=0.042、0.026)。单因素和多因素分析均提示对于CMV血清学阳性受者,供者CMV血清学阴性是CMV病毒反复激活、出血性膀胱炎的独立危险因素(P< 0.05)。移植后EBV D+/R+组和D-/R+组、D+/R-组和D-/R-组患者的EBV病毒血症的中位发作时间、EBV激活率、CMV与EBV共激活率、EBV反复激活率、cGVHD、PTLD、CMV肺炎发生率、2年OS率比较,差异均无统计学意义。移植后EBV D+/R+组患者的aGVHD发生率明显高于D-/R+组,D+/R-组患者的aGVHD发生率明显高于D-/R-组(P=0.001、0.001);D+/R+组患者移植后2年NRM明显高于D-/R+组,D+/R-组患者移植后2年NRM明显高于D-/R-组(P=0.004、0.033)。单因素和多因素分析均提示不论受者移植前EBV血清学状态如何,供者EBV血清学阳性是aGVHD和NRM的独立危险因素(P< 0.05)。结论 CMV-供者移植使CMV+受者CMV反复激活率、出血性膀胱炎发生率升高,接受CMV-供者移植可能会对CMV+受者临床预后产生不利影响。不论受者移植前EBV血清学状态如何,与供者EBV+组比较,供者EBV-组患者的aGVHD发生率和2年NRM低,移植患者可能受益于EBV血清学阴性供者。
-
关键词:
- 异基因造血干细胞移植 /
- EB病毒 /
- 巨细胞病毒 /
- 血清学状态 /
- 临床预后
Abstract: Objective To investigate whether the serologic status of CMV and EBV in donors affects CMV and EBV activation, clinical features and prognosis in patients after allogeneic hematopoietic stem cell transplantation(allo-HSCT).Methods A total of 215 patients underwent allo-HSCT and corresponding donors from September 2017 to September 2020 in our hospital were included. The serological status of CMV and EBV of donors and recipients was counted, and the number of CMV and EBV DNA copies of patients after transplantation was continuously monitored for at least 6 months, and the incidence of CMV and EBV viremia and clinical follow-up data were analyzed by χ2test, Kaplan-Meier method and Cox regression model.Results In patients with CMV D+/R+ and D-/R+, D+/R-and D-/R-group after transplantation, there was no significant difference in median incidence time of CMV viremia, median number of highest viral copies of CMV, co-activation rate of CMV and EBV, CMV activation rate, persistent positive rate of CMV virus, incidence of acute graft-versus-host disease(aGVHD) after transplantation, chronic graft-versus-host disease(cGVHD), lymphoproliferative disorders(PTLD) after transplantation, CMV pneumonia, 2-year survival(OS) rate, non-relapsed death mortality(NRM). The CMV virus re-activation rate and the incidence of hemorrhagic cystitis in D-/R+ group were significantly higher than those in D+/R+ group(P=0.042, 0.026). Both univariate and multivariate analysis suggested that CMV negative donors was an independent risk factor for recurrent CMV activation and hemorrhagic cystitis in CMV seropositivity recipients(P< 0.05). There was no significant difference in median onset time of EBV viremia, EBV activation rate, co-activation rate of CMV and EBV, recurrent EBV activation rate, cGVHD, CMV pneumonia, PTLD incidence, and 2-year OS rate in patients with EBV D+/R+ and D-/R+, D+/R-and D-/R-group after transplantation. The incidence of aGVHD was significantly higher in D+/R+ group than that in D-/R+ group, and the incidence of aGVHD in D+/R-group was significantly higher than that in D-/R-group(P=0.001, 0.001). The 2-year NRM after transplantation in D+/R+ group was significantly higher than that in D-/R+ group, and the 2-year NRM after transplantation in D+/R-group was significantly higher than that in D-/R-group(P=0.004, 0.033). Both univariate and multivariate analysis suggested that seropositive donors of EBV was an independent risk factor for aGVHD and NRM, regardless of recipients' s serological status before transplantation(P< 0.05).Conclusion CMV- donor transplantation increases the rate of CMV recurrent activation, and the incidence of hemorrhagic cystitis, and receiving CMV- donor transplantation may adversely affect the clinical prognosis of CMV+ recipients. Regardless of the recipient's serological status before transplantation, patients in the donor EBV- group have a low incidence of aGVHD and 2-year NRM compared to the donor EBV+ group, and transplant patients may benefit from an EBV- negative donor. -

-
表 1 供者CMV血清学状态对移植患者临床结果的影响
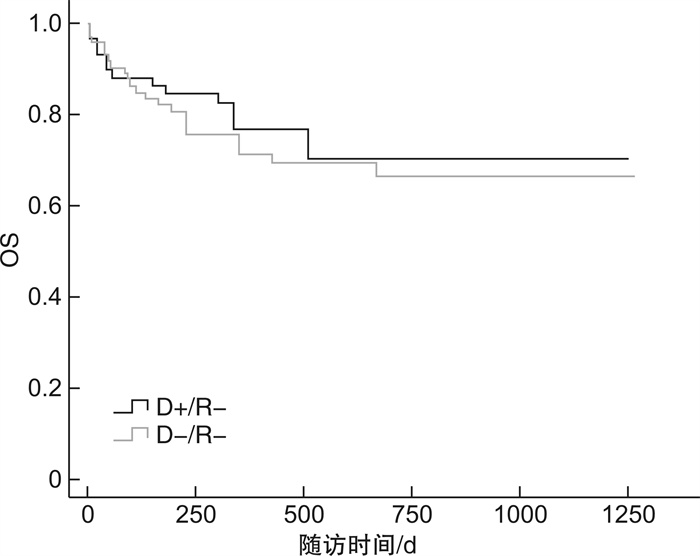
临床特征 D+/R+ D-R+ P D+/R- D-R- P 例数 35 48 59 73 EBV与CMV共激活/例(%) 4(11.4) 4(8.3) 0.637 6(10.2) 14(19.2) 0.151 CMV激活/例(%) 12(34.3) 26(54.2) 0.064 22(37.3) 30(41.1) 0.962 CMV发作时间/d 35(14~62) 33(2~135) 0.295 34(13~250) 38(2~989) 0.766 CMV病毒拷贝量/(copies·mL-1) 1.08×104 1.84×104 0.173 5.84×103 6.85×103 0.202 CMV持续阳性/例(%) 6(17.1) 15(31.3) 0.079 10(16.9) 14(19.2) 0.873 CMV激活次数>2次/例(%) 4(11.4) 14(29.2) 0.042 14(23.7) 12(16.4) 0.233 aGVHD/例(%) 6(17.1) 13(27.1) 0.257 13(22.0) 14(19.2) 0.386 cGVHD/例(%) 5(14.3) 13(27.1) 0.117 10(16.9) 18(24.7) 0.716 CMV肺炎/例(%) 4(11.4) 3(6.3) 0.495 0 5(6.8) 0.965 出血性膀胱炎/例(%) 3(8.6) 15(31.3) 0.026 5(8.5) 20(27.4) 0.077 PTLD/例(%) 2(5.7) 2(4.2) 0.556 1(1.7) 3(4.1) 0.869 存活/例(%) 27(77.1) 29(60.4) 0.149 45(76.3) 52(71.2) 0.639 表 2 供者CMV血清学状态对移植并发症、OS及NRM影响的单因素分析
供者CMV血清学状态 D+/R+ vs D-/R+ D+/R- vs D-/R- HR(95%CI) P HR(95%CI) P CMV激活次数>2次 3.192(1.041~9.786) 0.042 1.641(0.727~3.704) 0.233 aGVHD 1.753(0.664~4.627) 0.257 1.401(0.653~3.004) 0.386 cGVHD 2.288(0.812~6.441) 0.117 0.865(0.396~1.889) 0.716 CMV肺炎 0.593(0.132~2.660) 0.495 0 0.965 出血性膀胱炎 4.115(1.184~14.304) 0.026 0.409(0.152~1.100) 0.077 PTLD 0.486(0.044~5.371) 0.556 0.860(0.143~5.179) 0.869 OS 1.840(0.804~4.209) 0.149 0.850(0.432~1.675) 0.639 NRM 1.971(0.727~5.341) 0.182 0.791(0.342~1.831) 0.584 表 3 供者EBV血清学状态对移植患者临床结果的影响
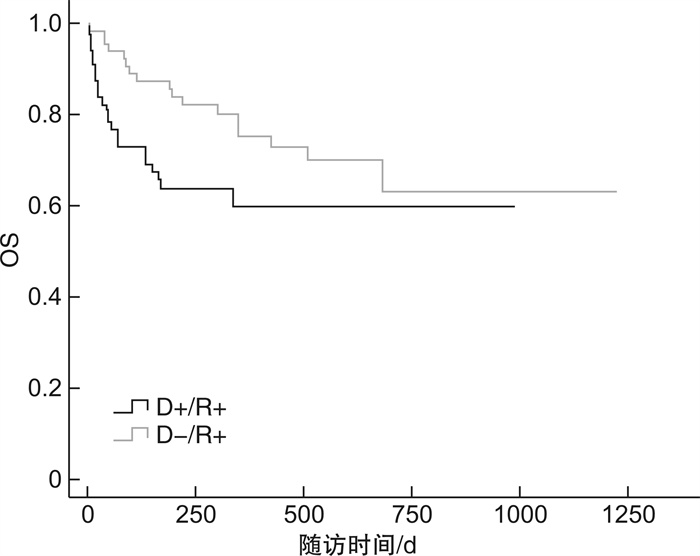
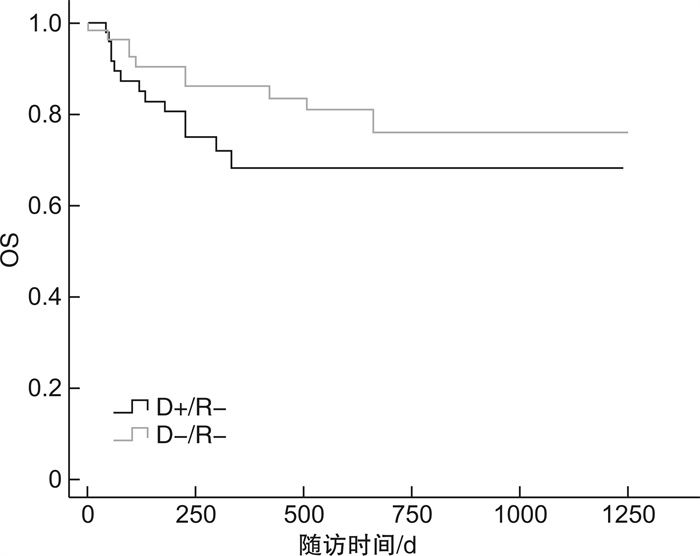
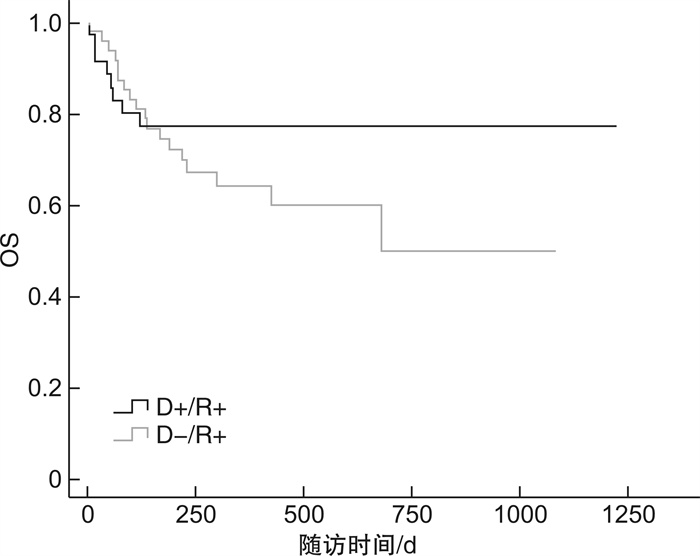
临床特征 D+/R+ D-/R+ P D+/R- D-/R- P 例数 55 62 46 52 EBV激活/例(%) 30(54.5) 36(58.1) 0.702 29(63.0) 26(50.0) 0.194 EBV与CMV共激活/例(%) 10(18.2) 6(9.7) 0.181 6(13.0) 6(11.5) 0.821 EBV发作时间/d 33(2~55) 43(19~150) 0.417 40(16~188) 52(14~163) 0.333 EBV病毒拷贝量/(copies·mL-1) 1.11×105 3.78×104 0.160 4.29×104 3.15×104 0.796 EBV持续阳性/例(%) 26(47.3) 22(35.5) 0.196 22(47.8) 14(26.9) 0.869 EBV激活次数>2次/例(%) 18(32.7) 19(30.6) 0.809 14(30.4) 13(25.0) 0.548 aGVHD/例(%) 17(30.9) 11(17.7) 0.001 16(34.8) 2(3.8) 0.001 cGVHD/例(%) 6(10.9) 16(25.8) 0.922 8(17.4) 16(30.8) 0.438 CMV肺炎/例(%) 5(9.1) 1(1.6) 0.119 3(6.5) 3(5.8) 0.404 PTLD/例(%) 2(3.6) 0 0.965 3(6.5) 3(5.8) 0.503 存活/例(%) 34(61.8) 44(71.0) 0.210 33(71.7) 42(80.8) 0.070 表 4 供者EBV血清学状态对移植并发症、OS及NRM影响的单因素分析
供者EBV血清学状态 D+/R+ vs D-/R+ D+/R -vs D-/R- HR(95%CI) P HR(95%CI) P aGVHD 0.283(0.130~0.613) 0.001 11.455(2.631~49.877) 0.001 cGVHD 1.049(0.403~2.733) 0.922 0.714(0.305~1.673) 0.438 CMV肺炎 0.257(0.047~1.419) 0.119 2.146(0.357~12.893) 0.404 PTLD 0 0.965 1.731(0.348~8.614) 0.503 OS 1.697(0.743~3.876) 0.210 0.557(0.295~1.049) 0.070 NRM 0.299(0.130~0.687) 0.004 4.069(1.118~14.809) 0.033 表 5 供者CMV和EBV血清学状态对移植并发症、NRM影响的多因素分析
指标 HR(95%CI) P 供者CMV血清学状态 D+/R+ vs D-/R+ CMV激活次数>2次 4.442(1.416~13.936) 0.011 出血性膀胱炎 7.992(2.118~30.162) 0.002 供者EBV血清学状态 D+/R+ vs D-/R+ aGVHD 0.288(0.130~0.639) 0.002 NRM 0.343(0.142~0.828) 0.017 D+/R- vs D-/R- aGVHD 11.114(2.528~48.862) 0.001 NRM 3.828(1.017~14.405) 0.047 -
[1] 於芳芳, 杨隽, 姜杰玲, 等. 异基因造血干细胞移植治疗骨髓增生异常综合征49例临床分析[J]. 临床血液学杂志, 2020, 33(1): 44-48. https://t.cnki.net/kcms/detail?v=znUxuWmAUtfhTqorxo61Gf94u6L0T3Jh19TrU-ohMDVEawmUqByczq3EXwOXnbnNiLewuL6FJOas2in3TOYuKdx0ijUZu6K8Ewd8xjwh8TjejneOhfNPrS7_kqXE52Ht&uniplatform=NZKPT
[2] Yasuda S, NajimaY, Konishi T, et al. Outcome of allogeneic hematopoietic stem cell transplantation for T-cell lymphoblastic leukemia/lymphoma: a single-center study[J]. Leuk Res, 2021, 108: 106627. doi: 10.1016/j.leukres.2021.106627
[3] Wiriyachai T, Chaya W, Anurathapan U, et al. Association between adenovirus infection and mortality outcome among pediatric patients after hematopoietic stem cell transplant[J]. Transpl Infect Dis, 2021, 23(6): e13742.
[4] Tsoumakas K, Giamaiou K, Goussetis E, et al. Epidemiology of viral infections among children undergoing hematopoietic stem cell transplant: a prospective single-center study[J]. Transpl Infect Dis, 2019, 21(4): 13095.
[5] Cho SY, Lee DG, Kim HJ. Cytomegalovirus infections after hematopoietic stem cell transplantation: current status and future immunotherapy[J]. Int J Mol Sci, 2019, 20(11): 2666. doi: 10.3390/ijms20112666
[6] Slade M, Goldsmith S, Romee R, et al. Epidemiology of infections following haploidentical peripheral blood hematopoietic cell transplantation[J]. Transpl Infect Dis, 2017, 19(1): 12629. doi: 10.1111/tid.12629
[7] Kolodziejczak M, Gil L, de la Camara R, et al. Impact of donor and recipient Epstein-Barr Virus serostatus on outcomes of allogeneic hematopoietic cell transplantation: a systematic review and meta-analysis[J]. Ann Hematol, 2021, 100(3): 763-777. doi: 10.1007/s00277-021-04428-9
[8] Solomon SR, Aubrey MT, Zhang X, et al. Selecting the best donor for haploidentical transplant: impact of HLA, killer cell immunoglobulin-like receptor genotyping, and other clinical variables[J]. Biol Blood Marrow Transplant, 2018, 24(4): 789-798. doi: 10.1016/j.bbmt.2018.01.013
[9] 刘静, 付强, 王昱, 等. 供者巨细胞病毒血清学阴性状态对异基因造血干细胞移植患者预后影响的临床分析[J]. 中华内科杂志, 2021, 60(5): 459-465. doi: 10.3760/cma.j.cn112138-20200714-00668
[10] Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors[J]. Transplantation, 1974, 18(4): 295-304. doi: 10.1097/00007890-197410000-00001
[11] Martin PJ, Lee SJ, Przepiorka D, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: VI. The 2014 Clinical Trial Design Working Group Report[J]. Biol Blood Marrow Transplant, 2015, 21(8): 1343-1359. doi: 10.1016/j.bbmt.2015.05.004
[12] Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report[J]. Biol Blood Marrow Transplant, 2005, 11(12): 945-956.
[13] Ljungman P, Boeckh M, Hirsch HH, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials[J]. Clin Infect Dis, 2017, 64(1): 87-91. doi: 10.1093/cid/ciw668
[14] 赵晓盨, 刘代红, 许兰平, 等. 异基因造血干细胞移植后巨细胞病毒肺炎临床特点分析[J]. 北京大学学报(医学版), 2009, 41(5): 548-553. doi: 10.3969/j.issn.1671-167X.2009.05.009
[15] 谭栩, 张璇, 高力, 等. 更昔洛韦和膦甲酸钠预防异基因造血干细胞移植后巨细胞病毒感染效果比较的单中心、前瞻性随机对照研究[J]. 临床血液学杂志, 2020, 33(9): 618-624. https://t.cnki.net/kcms/detail?v=znUxuWmAUtfKDmeQavf3aUZAc7gnzkvbp8_L2szHIFfIMYu0OzXtf7S6WQ5aPlS_VxbAJ42yT6b0sOcTBdUx9crOTF5dHF_MDhKJq4-YjKvpGcv_UNSzBv55W6AeYvo9&uniplatform=NZKPT
[16] Naik S, Riches M, Hari P, et al. Survival outcomes of allogeneic hematopoietic cell transplants with EBV-positive or EBV-negative post-transplant lymphoproliferative disorder, a cibmtr study[J]. Transpl Infect Dis, 2019, 21(5): e13145.
[17] Ding YY, Ru YH, Song TM, et al. Epstein-Barr virus and cytomegalovirus reactivation after allogeneic hematopoietic cell transplantation in patients with non-Hodgkin lymphoma: the prevalence and impacts on outcomes: EBV and CMV reactivation post allo-HCT in NHL[J]. Ann Hematol, 2021, 100(11): 2773-2785. doi: 10.1007/s00277-021-04642-5
[18] Manuel O, Avery RK. Update on cytomegalovirus in transplant recipients: new agents, prophylaxis, and cell-mediated immunity[J]. Curr Opin Infect Dis, 2021, 34(4): 307-313.
[19] Czyzewski K, Dziedzic M, Salamonowicz M, et al. Epidemiology, outcome and risk factors analysis of viral infections in children and adolescents undergoing hematopoietic cell transplantation: antiviral drugs do not prevent Epstein-Barr virus reactivation[J]. Infect Drug Resist, 2019, 12: 3893-3902. doi: 10.2147/IDR.S224291
[20] Lindsay J, Othman J, Heldman MR, et al. Epstein-Barr virus posttransplant lymphoproliferative disorder: update on management and outcomes[J]. Curr Opin Infect Dis, 2021, 34(6): 635-645. doi: 10.1097/QCO.0000000000000787
[21] Yong MK, Cameron PU, Slavin M, et al. Identifying cytomegalovirus complications using the quantiferon-CMV assay after allogeneic hematopoietic stem cell transplantation[J]. J Infect Dis, 2017, 215(11): 1684-1694. doi: 10.1093/infdis/jix192
[22] Ganepola S, Gentilini C, Hilbers U, et al. Patients at high risk for CMV infection and disease show delayed CD8+ T-cell immune recovery after allogeneic stem cell transplantation[J]. Bone Marrow Transplant, 2007, 39(5): 293-299. doi: 10.1038/sj.bmt.1705585
[23] Nichols WG, Corey L, Gooley T, et al. High risk of death due to bacterial and fungal infection among cytomegalovirus(CMV)-seronegative recipients of stem cell transplants from seropositive donors: evidence for indirect effects of primary CMV infection[J]. J Infect Dis, 2002, 185(3): 273-282. doi: 10.1086/338624
[24] Grob JP, Grundy JE, Prentice HG, et al. Immune donors can protect marrow-transplant recipients from severe cytomegalovirus infections[J]. Lancet, 1987, 1(8536): 774-776.
[25] Styczynski J, Tridello G, Gil L, et al. Prognostic impact of EBV serostatus in patients with lymphomas or chronic malignancies undergoing allogeneic HCT[J]. Bone Marrow Transplant, 2019, 54(12): 2060-2071. doi: 10.1038/s41409-019-0627-9
-




 下载:
下载: