Increment of prednisone dosage improves the response rate of myelofibrosis-associated anemia
-
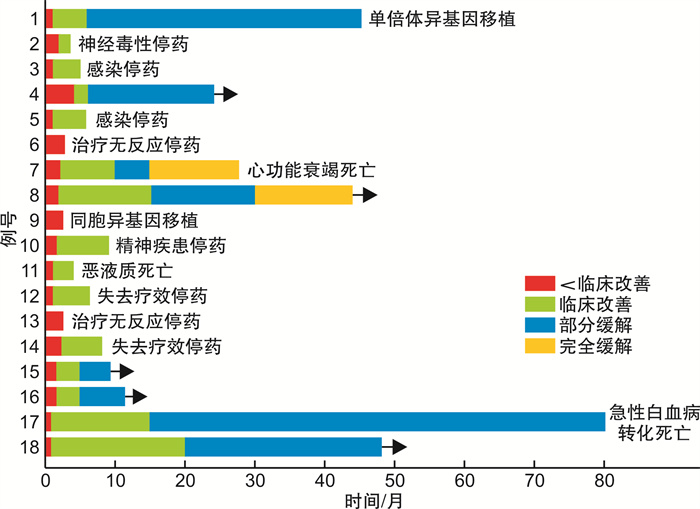
摘要: 目的 观察提高泼尼松剂量单药治疗骨髓纤维化贫血的疗效。方法 回顾性分析接受较高剂量泼尼松单药治疗20例伴有贫血的骨髓纤维化患者的临床资料,主要内容包括性别、年龄、诊断、外周血、骨髓形态学、免疫学、细胞遗传学、基因突变、骨髓活检、肝脾肿大、体质性症状及既往治疗情况。结果 20例患者中男16例、女4例,中位年龄57(45,68)岁,治疗前中位血红蛋白水平60(55,80)g/L,口服泼尼松0.7~1.0 mg/(kg·d)[中位剂量0.8 mg/(kg·d)]至少3个月,75.0%(15/20)患者经过中位起效时间3(2,8)周的治疗获得临床反应,中位疗效持续时间为19(6,45)个月。非输血依赖10例患者中,8例患者血红蛋白上升大于20 g/L; 10例输血依赖患者中,2例患者治疗有效但因不良反应无法耐受早期停药,其余8例输血依赖患者中7例摆脱输血依赖。根据IWG-MRT关于贫血的疗效评价标准,2例患者获得完全缓解,6例患者获得部分缓解,7例患者获得临床改善。20例患者均未见明显的血液学不良反应; 非血液学不良反应主要为继发感染和神经毒性。结论 增加泼尼松剂量[中位剂量0.8 mg/(kg·d)]持续服用至少3个月,能够提高骨髓纤维化患者贫血治疗反应率,改善患者的生活质量。Abstract: Objective To evaluate the efficacy of corticosteroid monotherapy through increment of prednisone dosage on myelofibrosis-associated anemia.Methods The outcome and clinical data of 20 myelofibrosis patients treated with medium-dose prednisone were retrospectively analyzed, including gender, age, diagnosis, whole blood count, bone marrow examination, immunophenotyping, cytogenetics changes, somatic mutations, bone marrow biopsy, hepatomegaly and/or splenomegaly, constitutional symptoms and prior treatment.Results Among the 20 patients, there were 16 males and 4 females, with a median age of 57(45, 68) years. Before treatment, the median hemoglobin level was 60(55, 80) g/L. Oral prednisone dose of 0.7-1.0 mg/(kg·d), median dose of 0.8 mg/(kg·d) was taken for at least 3 months. Fifteen patients(75.0%) obtained clinical response after treatment with a median onset time of 3(2, 8) weeks. The median duration of efficacy was 19(6, 45) months. Among the 10 transfusion-independent patients, the hemoglobin increased by more than 20 g/L in 8 patients. Among the 10 transfusion-dependent patients, 2 cases were effective but could not tolerate early withdrawal due to adverse events; among the other 8 transfusion-dependent patients, 7 cases got rid of blood transfusion dependence. According to the revised International Working Group for Myelofibrosis Research and Treatment(IWG-MRT) criteria, 2 cases achieved complete remission, 6 cases achieved partial remission and 7 cases achieved clinical improvement. There were no obvious hematological adverse events in 20 patients. Non hematological adverse events were mainly secondary infection and neurotoxicity.Conclusion Increment of prednisone dosage, with a median dose of 0.8 mg/(kg·d) continuously at last 3 months results in higher response rate of myelofibrosis-associated anemia, together with improved quality of life.
-
Key words:
- myelofibrosis /
- increment of prednisone dosage /
- anemia
-

-
表 1 增加泼尼松剂量后治疗有反应的15例患者治疗前后临床数据比较
患者编号 年龄/岁 性别 骨髓纤维化类型 治疗前后血红蛋白(最好的血红蛋白反应)/(g·L-1) 起效时间/周 持续时间/月 1 37 男 血小板增多症后继发 90 vs 115 2 45 2 50 男 原发 输血依赖vs 90 2 5 3 27 男 原发 74 vs 135 4 35+ 4 42 男 血小板增多症后继发 输血依赖vs 83 3 6 5 62 男 原发 输血依赖vs 91 9 27 6 47 男 原发 69 vs 91 8 55+ 7 78 男 原发 66 vs 89 3 4 8 65 男 血小板增多症后继发 输血依赖vs 86 3 6 9 60 男 原发 输血依赖vs 91 8 4 10 57 男 原发 70 vs 115 4 19+ 11 49 男 原发 78 vs 118 4 24+ 12 45 女 原发 65 vs 115 2 80 13 61 男 原发 71 vs 105 2 59+ 14 50 女 原发 输血依赖vs 90 3 10+ 15 66 男 血小板增多症后继发 输血依赖vs 72 2 8+ -
[1] Tefferi A. Primary myelofibrosis: 2021 update on diagnosis, risk-stratification and management[J]. Am J Hematol, 2021, 96(1): 145-162. doi: 10.1002/ajh.26050
[2] 白雪, 赵一帆, 冯志金, 等. 早期或纤维化前原发性骨髓纤维化与原发性血小板增多症鉴别的研究进展[J]. 临床血液学杂志, 2021, 34(3): 220-224. https://t.cnki.net/kcms/detail?v=znUxuWmAUtftXi2dABZBx1XTNTRzApfqNtVngbcZOQlHcEX19oBO84S9Hpa3mGYTDHuWY6yAEX_h70r6yxIixGvMF8uKperUUKRKxgXQHYnqa3JNfnOo3BLY5pIQefZ7&uniplatform=NZKPT
[3] Asher S, McLornan DP, Harrison CN. Current and future therapies for myelofibrosis[J]. Blood Rev, 2020, 42: 100715. doi: 10.1016/j.blre.2020.100715
[4] Naymagon L, Mascarenhas J. Myelofibrosis-Related Anemia: Current and Emerging Therapeutic Strategies[J]. Hemasphere, 2017, 1(1): e1. doi: 10.1097/HS9.0000000000000001
[5] Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia[J]. Blood, 2016, 127(20): 2391-2405. doi: 10.1182/blood-2016-03-643544
[6] Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm(MPN)symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs[J]. J Clin Oncol, 2012, 30(33): 4098. doi: 10.1200/JCO.2012.42.3863
[7] Gangat N, Caramazza D, Vaidya R, et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status[J]. J Clin Oncol, 2010, 29(4): 392-397.
[8] Tefferi A, Cervantes F, Mesa R, et al. Revised response criteria for myelofibrosis: International Working Group-Myeloproliferative Neoplasms Research and Treatment(IWG-MRT)and European LeukemiaNet(ELN)consensus report[J]. Blood, 2013, 122(8): 1395-1398. doi: 10.1182/blood-2013-03-488098
[9] Mesa RA, Yao X, Cripe LD, et al. Lenalidomide and prednisone for myelofbrosis: Eastern Cooperative Oncology Group(ECOG)phase 2 trial E4903[J]. Blood, 2010, 116(22): 4436-4438. doi: 10.1182/blood-2010-05-287417
[10] Chihara D, Masarova L, Newberry KJ, et al. Long-term results of a phase Ⅱ trial of lenalidomide plus prednisone therapy for patients with myelofibrosis[J]. Leuk Res, 2016, 48: 1-5. doi: 10.1016/j.leukres.2016.06.007
[11] Castillo-Tokumori F, Chetasi T, Najla A, et al. Retrospective analysis of the clinical use and beneft of lenalidomide and thalidomide in myelofbrosis[J]. Clin Lymphoma Myeloma Leuk, 2020, 20(12): e956-e960. doi: 10.1016/j.clml.2020.07.006
[12] Daver N, Shastri A, Kadia T, et al. Phase Ⅱ study of pomalidomide in combination with prednisone in patients with myelofibrosis and significant anemia[J]. Leuk Res, 2014, 38(9): 1126-1129. doi: 10.1016/j.leukres.2014.06.015
[13] Tefferi A, Al-Ali HK, Barosi G, et al. A randomized study of pomalidomide vs placebo in persons with myeloproliferative neoplasm-associated myelofbrosis and RBC-transfusion dependence[J]. Leukemia, 2017, 31(4): 896-902. doi: 10.1038/leu.2016.300
[14] Hernández-Boluda JC, Martínez-Trillos A, García-Gutiérrez V, et al. Long-term results of prednisone treatment for the anemia of myelofibrosis[J]. Leuk Lymphoma, 2016, 57(1): 120-124. doi: 10.3109/10428194.2015.1046866
[15] Bělohlávková P, Maisnar V, Voglová J, et al. Improvement of Anaemia in Patients with Primary Myelofibrosis by Low-Dose Thalidomide and Prednisone[J]. Acta Medica(Hradec Kralove), 2016, 59(2): 50-53.
[16] Thapaliya P, Tefferi A, Pardanani A. International working group for myelofibrosis research and treatment response assessment and long-term follow-up of 50 myelofibrosis patients treated with thalidomide-prednisone based regimens[J]. Am J Hematol, 2011, 86(1): 96-98. doi: 10.1002/ajh.21892
[17] 肖超, 宋陆茜, 许峰, 等. 芦可替尼联合TCP方案治疗骨髓纤维化患者的真实世界研究[J]. 临床血液学杂志, 2020, 33(11): 753-758. https://t.cnki.net/kcms/detail?v=znUxuWmAUtdYVuigdRKkkCQj50p05akdzT3bzYO1ME3gyAgLaOv6elp0TS4OZV9mGPQLWuyFdNOjH0uE8qt-QztzQr_o0gjZcBmLhVR2UaaXwxwE5VYdOyqdCtXB0V3F&uniplatform=NZKPT
[18] Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis[J]. N Engl J Med, 2012, 366(9): 787-798. doi: 10.1056/NEJMoa1110556
[19] Bergmann TK, Barraclough KA, Lee KJ, et al. Clinical pharmacokinetics and pharmacodynamics of prednisolone and prednisone in solid organ transplantation[J]. Clin Pharmacokinet, 2012, 51(11): 711-741. doi: 10.1007/s40262-012-0007-8
[20] Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs[J]. N Engl J Med, 2005, 353(16): 1711-1712. doi: 10.1056/NEJMra050541
[21] Barosi G, Magrini U, Gale RP. Does auto-immunity contribute to anemia in myeloproliferative neoplasms(MPN)-associated myelofibrosis[J]. Leuk Res, 2010, 34(9): 1119-1120. doi: 10.1016/j.leukres.2010.05.010
[22] Meijer OC, Koorneef LL, Kroon J. Glucocorticoid receptor modulators[J]. Ann Endocrinol(Paris), 2018, 79(3): 107-111. doi: 10.1016/j.ando.2018.03.004
[23] 黄晓军. 血液病/恶性肿瘤患者侵袭性真菌病的诊断标准与治疗原则(第六次修订版)[J]. 中华内科杂志, 2020, 59(10): 754-763. doi: 10.3760/cma.j.cn112138-20200627-00624
[24] Perrot S, Le Jeunne C. Atteinte musculaire et glucocorticoi des[Steroid-induced myopathy][J]. Presse Med, 2012, 41(4): 422-426. doi: 10.1016/j.lpm.2012.01.004
-




 下载:
下载: