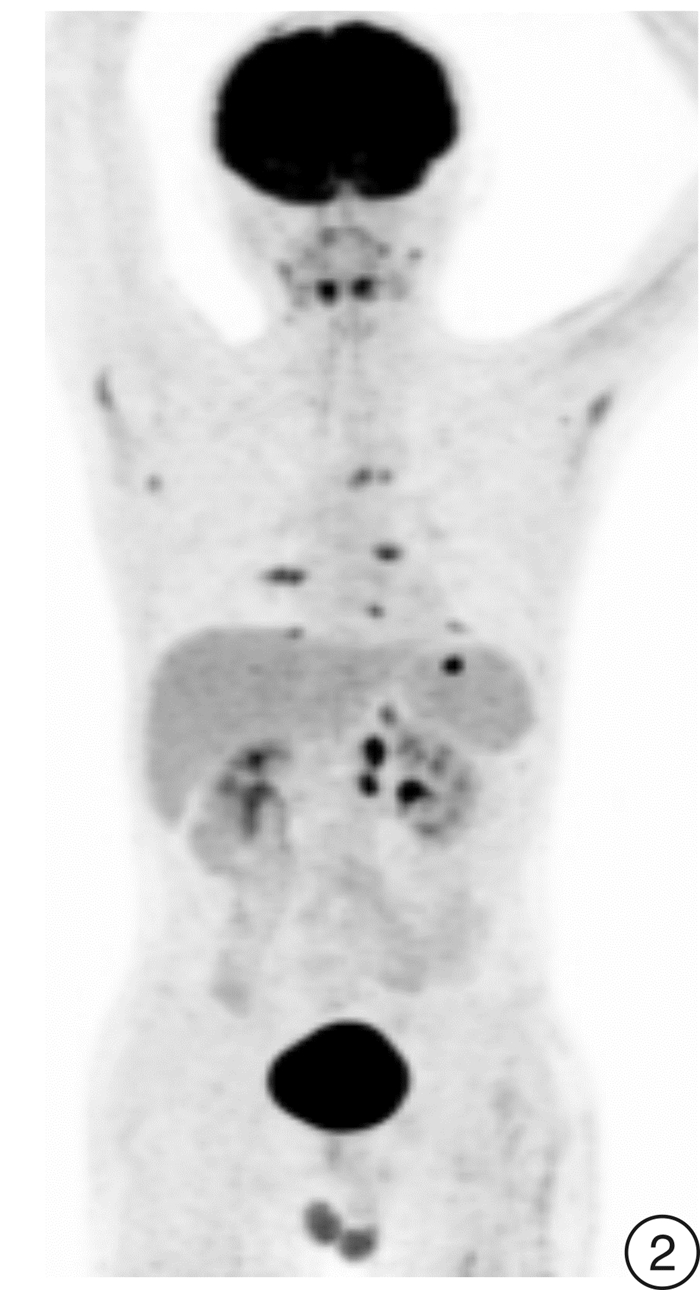
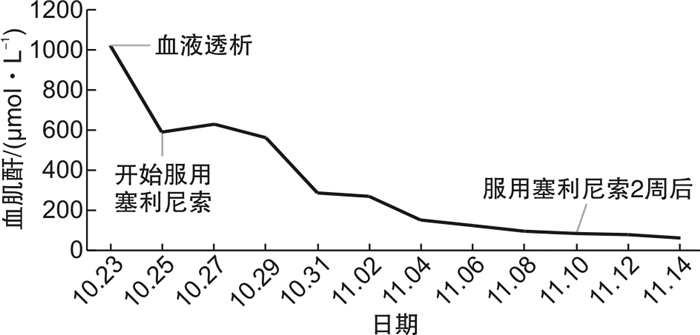
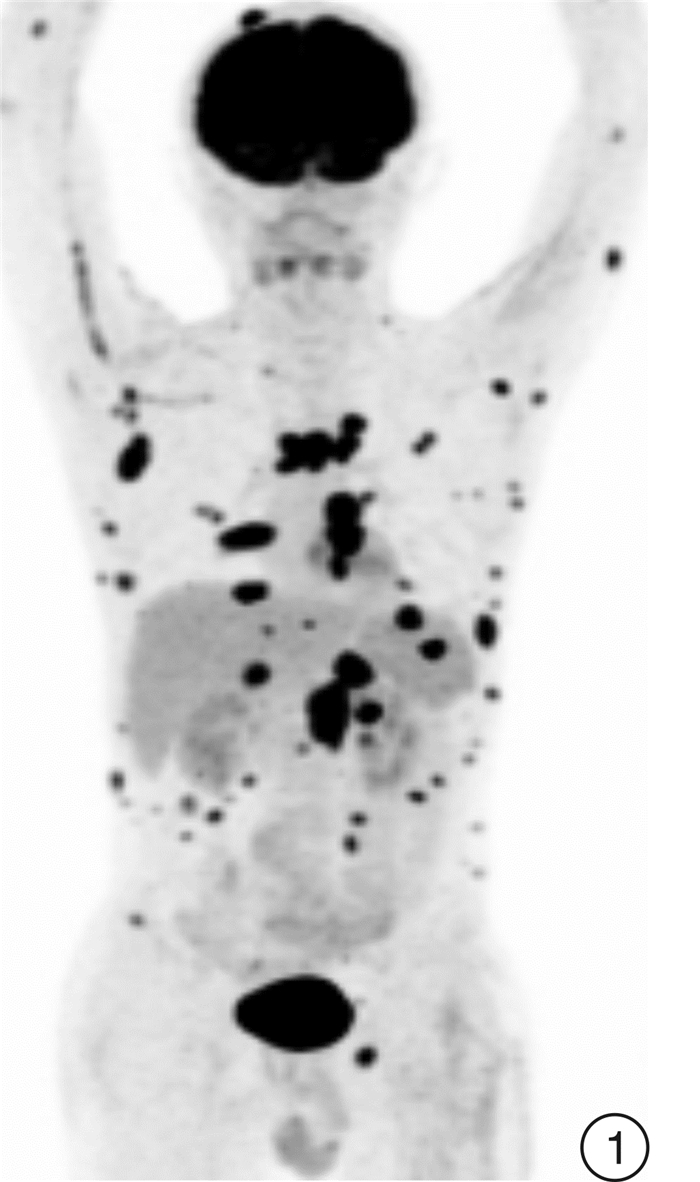
Selinexor-based chemotherapy in the treatment of two patients with relapsed/refractory plasmablastic lymphoma
-
摘要: 浆母细胞淋巴瘤(PBL)是一种罕见的侵袭性非霍奇金淋巴瘤,其预后不容乐观,总生存期较短,目前尚无标准的治疗方法。塞利尼索是全球首款选择性核输出蛋白抑制剂,它选择性地结合核输出蛋白1,促使癌细胞周期阻滞和凋亡。塞利尼索是一种很有前途的癌症治疗方法,目前已被批准用于治疗多发性骨髓瘤和弥漫大B细胞淋巴瘤。本文结合具体的病例,探讨塞利尼索改善PBL治疗现状的可能。2例年轻、既往接受过多线化疗及放疗的PBL患者,其中1例应用塞利尼索联合GDP方案获得非常好的部分缓解,另外1例急性肾衰竭、已无化疗指征的患者,应用塞利尼索联合GDP方案后肾功能逐渐恢复正常。表明塞利尼索可能为PBL患者带来临床获益,有待积累更多病例验证疗效。Abstract: Plasmablastic lymphoma(PBL) is an uncommon and aggressive type of non-Hodgkin lymphoma. Its prognosis remains dismal with short overall survival and currently, there is no standard therapy for this entity. Selinexor is a first-in-class, novel selective nuclear export inhibitor that selectively binds to export protein 1, leading to cell cycle arrest and apoptosis. Selinexor represents a promising therapy in cancer treatment and has been approved for treating multiple myeloma and diffuse large B-cell lymphoma. Combined with specific cases, this paper discusses the possibility of selinexor to improve the current situation of PBL treatment. There were two young patients with PBL who had received multiple prior lines of chemotherapy and radiotherapy. One patient obtained a very good partial response with selinexor in combination with the GDP regimen, and the other patient with acute renal failure and no indication for chemotherapy experienced renal function recovery through the same regimen. It indicates that selinexor may bring clinical benefits to PBL patients, and more cases are needed to verify the efficacy.
-
Key words:
- selinexor /
- exportin-1 /
- relapsed/refractory /
- plasmablastic lymphoma
-

-
[1] Lopez A, Abrisqueta P. Plasmablastic lymphoma: current perspectives[J]. Blood Lymphat Cancer, 2018, 8: 63-70.
[2] Pileri SA, Mazzara S, Derenzini E. Plasmablastic lymphoma: one or more tumors?[J]. Haematologica, 2021, 106(10): 2542-2543. doi: 10.3324/haematol.2021.278841
[3] Azmi AS, Uddin MH, Mohammad RM. The nuclear export protein XPO1-from biology to targeted therapy[J]. Nat Rev Clin Oncol, 2021, 18(3): 152-169. doi: 10.1038/s41571-020-00442-4
[4] 阙伊湄, 徐孟磊, 王迪, 等. Selinexor联合化疗在CAR-T治疗后进展的多发性骨髓瘤中的应用探索[J]. 临床血液学杂志, 2021, 34(11): 815-818, 824. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXZ202111014.htm
[5] 伍世钢, 黄启智, 李进邦, 等. 浆母细胞淋巴瘤1例报告及文献回顾[J]. 现代肿瘤医学, 2019, 27(23): 4289-4292. https://www.cnki.com.cn/Article/CJFDTOTAL-SXZL201923041.htm
[6] Fonseca FP, Robinson L, van Heerden MB, et al. Oral plasmablastic lymphoma: A clinicopathological study of 113 cases[J]. J Oral Pathol Med, 2021, 50(6): 594-602. doi: 10.1111/jop.13210
[7] Tchernonog E, Faurie P, Coppo P, et al. Clinical characteristics and prognostic factors of plasmablastic lymphoma patients: analysis of 135 patients from the LYSA group[J]. Ann Oncol, 2017, 28(4): 843-848. doi: 10.1093/annonc/mdw684
[8] Li YJ, Li JW, Chen KL, et al. HIV-negative plasmablastic lymphoma: report of 8 cases and a comprehensive review of 394 published cases[J]. Blood Res, 2020, 55(1): 49-56. doi: 10.5045/br.2020.55.1.49
[9] Fernandez-Alvarez R, Gonzalez-Rodriguez AP, Rubio-Castro A, et al. Bortezomib plus CHOP for the treatment of HIV-associated plasmablastic lymphoma: clinical experience in three patients[J]. Leuk Lymphoma, 2016, 57(2): 463-466. doi: 10.3109/10428194.2015.1050666
[10] Castillo JJ, Guerrero-Garcia T, Baldini F, et al. Bortezomib plus EPOCH is effective as frontline treatment in patients with plasmablastic lymphoma[J]. Br J Haematol, 2019, 184(4): 679-682. doi: 10.1111/bjh.15156
[11] Dittus C, Grover N, Ellsworth S, et al. Bortezomib in combination with dose-adjusted EPOCH(etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin)induces long-term survival in patients with plasmablastic lymphoma: a retrospective analysis[J]. Leuk Lymphoma, 2018, 59(9): 2121-2127. doi: 10.1080/10428194.2017.1416365
[12] Schmit JM, DeLaune J, Norkin M, et al. A Case of Plasmablastic Lymphoma Achieving Complete Response and Durable Remission after Lenalidomide-Based Therapy[J]. Oncol Res Treat, 2017, 40(1-2): 46-48. doi: 10.1159/000455146
[13] Benkova K, Mihalyova J, Hajek R, et al. Selinexor, selective inhibitor of nuclear export: Unselective bullet for blood cancers[J]. Blood Rev, 2021, 46: 100758. doi: 10.1016/j.blre.2020.100758
[14] Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma[J]. Blood, 2015, 125(15): 2323-2330. doi: 10.1182/blood-2014-10-567479
[15] DeSisto JA, Flannery P, Lemma R, et al. Exportin 1 Inhibition Induces Nerve Growth Factor Receptor Expression to Inhibit the NF-κB Pathway in Preclinical Models of Pediatric High-Grade Glioma[J]. Mol Cancer Ther, 2020, 19(2): 540-551. doi: 10.1158/1535-7163.MCT-18-1319
[16] Gravina GL, Senapedis W, McCauley D, et al. Nucleo-cytoplasmic transport as a therapeutic target of cancer[J]. J Hematol Oncol, 2014, 7: 85. doi: 10.1186/s13045-014-0085-1
[17] Etchin J, Sun Q, Kentsis A, et al. Antileukemic activity of nuclear export inhibitors that spare normal hematopoietic cells[J]. Leukemia, 2013, 27(1): 66-74. doi: 10.1038/leu.2012.219
[18] Chari A, Vogl DT, Gavriatopoulou M, et al. Oral Selinexor-Dexamethasone for Triple-Class Refractory Multiple Myeloma[J]. N Engl J Med, 2019, 381(8): 727-738. doi: 10.1056/NEJMoa1903455
[19] Kalakonda N, Maerevoet M, Cavallo F, et al. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma(SADAL): a single-arm, multinational, multicentre, open-label, phase 2 trial[J]. Lancet Haematol, 2020, 7(7): e511-e522. doi: 10.1016/S2352-3026(20)30120-4
[20] Corno C, Stucchi S, De Cesare M, et al. FoxO-1 contributes to the efficacy of the combination of the XPO1 inhibitor selinexor and cisplatin in ovarian carcinoma preclinical models[J]. Biochem Pharmacol, 2018, 147: 93-103. doi: 10.1016/j.bcp.2017.11.009
[21] Azmi A, Khan HY, Muqbil I, et al. Preclinical Assessment with Clinical Validation of Selinexor with Gemcitabine and Nab-Paclitaxel for the Treatment of Pancreatic Ductal Adenocarcinoma[J]. Clin Cancer Res, 2019, 26(6): 1338-1348.
[22] Maerevoet M, Casasnovas O, Cartron G, et al. Selinexor in Combination with R-GDP for Patients with Relapsed/Refractory B-Cell Lymphoma: Results of the Selinda Phase Ib Lysa Study[C]. 2021 Dec. Presented at: 63rd ASH Annual Meeting and Exposition. Poster 1411.
[23] Lee ST, Pinto A, Yimer H, et al. A Phase 2/3, Multicenter Randomized Study of Rituximab-Gemcitabine-Dexamethasone-Platinum(R-GDP)with or without Selinexor in Patients with Relapsed/Refractory Diffuse Large B-Cell Lymphoma(RR DLBCL)[C]. 2021 Dec. Presented at: 63rd ASH Annual Meeting and Exposition. Poster 1420.
-




 下载:
下载: