Variation and significance of IL-2R, IL-6, IL-8 and TNF-α in diffuse large B-cell lymphoma
-
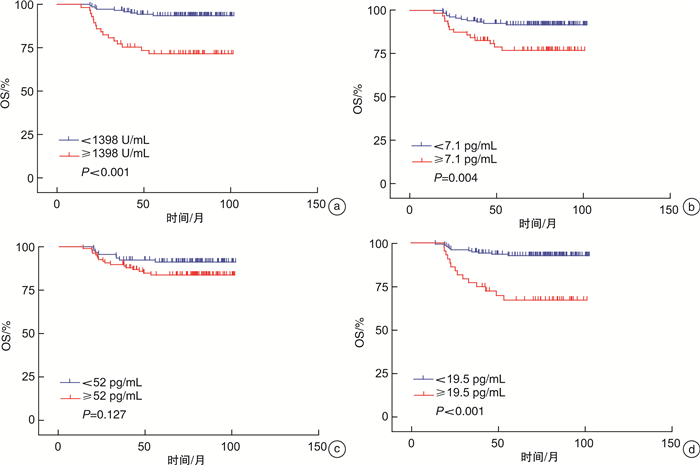
摘要: 目的 探究细胞因子IL-2R、IL-6、IL-8、TNF-α在弥漫性大B细胞淋巴瘤(DLBCL)中的变化及其意义。方法 收集上海交通大学医学院附属瑞金医院收治的155例R-CHOP(利妥昔单抗、环磷酰胺、阿霉素、长春新碱和泼尼松)方案治疗后达完全缓解且无进展生存期≥24个月的DLBCL患者(持续缓解组)和45例R-CHOP方案治疗后达完全缓解但是在2年内复发的DLBCL患者(复发组)。持续缓解组患者于治疗前、第6个周期治疗开始前以及持续缓解治疗结束1年后采血,复发组患者于治疗前、第6个周期治疗开始前及复发时间点采血。采用IMMUNITE 1000化学发光分析仪检测细胞因子IL-2R、IL-6、IL-8、TNF-α的含量。结果 IL-2R、IL-6及TNF-α含量在Ⅲ~Ⅳ期、ECOG评分≥2分、结外受累个数≥2个、IPI评分≥3分的DLBCL患者中显著升高(P<0.05);IL-8在IPI评分≥3分和双表达淋巴瘤(DEL)的患者中显著升高(P<0.05);IL-6在non-GCB亚型中显著升高(P<0.05)。复发组患者治疗前血清细胞因子IL-2R、IL-6、TNF-α含量均较持续缓解组显著升高(P<0.05)。治疗6个周期开始前同治疗前比较,复发组和持续缓解组IL-2R、IL-6、IL-8、TNF-α含量均显著降低(P<0.05),以上指标在持续缓解组持续降低,而复发组在复发时又显著升高(P<0.05)。IL-2R含量≥1398 U/mL(P<0.001)、IL-6含量≥7.1 pg/mL(P=0.004)、TNF-α含量≥19.5 pg/mL(P<0.001)与短的总生存期显著相关。结论 治疗前复发组和持续缓解组患者IL-2R、IL-6、IL-8、TNF-α含量存在明显差异,且以上指标在缓解时降低,复发时再次升高,或许可成为预测和评估DLBCL患者R-CHOP治疗后复发的有效血清标记分子。此外,治疗前血清中高的IL-2R、IL-6及TNF-α与DLBCL患者不良预后相关。Abstract: Objective To investigate the variation and significance of IL-2R, IL-6, IL-8 and TNF-α levels in diffuse large B-cell lymphoma(DLBCL).Methods A total of 155 cases of DLBCL with complete remission and progression free survival ≥ 24 months after R-CHOP treatment(continuous remission group) and 45 cases of relapsed DLBCL within 2 years after R-CHOP treatment(relapse group) were collected from Rui Jin Hospital, Shanghai Jiao Tong University School of Medicine. Blood samples were collected from patients of the continuous remission group at 3 time points, before treatment, before the start of the 6th cycle of treatment and one year after the end of continuous remission. Patients in the relapse group were sampled before treatment, before the start of the 6th cycle of treatment and at the time of relapse. The contents of inflammatory cytokines IL-2R, IL-6, IL-8 and TNF-α were detected by IMMUNITE 1000 chemiluminescence analyzer.Results The levels of IL-2R, IL-6 and TNF-α were significantly increased in DLBCL patients with Ann Arbor stage of Ⅲ-Ⅳ, ECOG score≥2, number of extranodal involvement≥2, IPI score≥3 (P < 0.05), while IL-8 level was significantly increased in patients with IPI score≥3 and DEL(P < 0.05) and IL-6 was significantly increased in non-GCB subtype(P < 0.05). The levels of serum cytokines IL-2R, IL-6 and TNF-α in the relapse group were significantly higher than those of the continuous remission group before treatment(P < 0.05). Compared with those before treatment, IL-2R, IL-6, IL-8 and TNF-α levels in the relapse group and the continuous remission group were significantly decreased before the start of the 6th cycle of treatment(P < 0.05), and the levels of the above cytokines continued to decreased in the continuous remission group while increased in the relapse group(P < 0.05). IL-2R content≥1398 U/mL(P < 0.001), IL-6 content ≥7.1 pg/mL(P=0.004), TNF-α content ≥19.5 pg/mL(P < 0.001) were significantly associated with shorter overall survival.Conclusion The levels of IL-2R, IL-6, IL-8 and TNF-α were significantly different between the relapsed group and the continuous remission group before treatment, and these cytokines levels were obviously decreased following remission while increased again when recurrence, which may be effective serum markers for predicting and evaluating the recurrence of DLBCL patients after R-CHOP treatment. In addition, high levels of serum IL-2R, IL-6, and TNF-α before initiation of treatment were associated with poor prognosis in DLBCL patients.
-
Key words:
- diffuse large-B cell lymphoma /
- continuous remission /
- relapse /
- IL-2R /
- IL-6 /
- IL-8 /
- TNF-α
-

-
表 1 不同临床病理特征DLBCL患者的血清细胞因子含量比较
M(P25,P75) 临床特征 IL-2R/(U·mL-1) IL-6/(pg·mL-1) IL-8 /(pg·mL-1) TNF-α/(pg·mL-1) Ann Arbor分期 Ⅰ~Ⅱ(n=121) 484.0(369.0,748.0) 3.1(2.0,6.1) 51.6(26.1,142.0) 8.8(6.6,10.9) Ⅲ~Ⅳ(n=79) 1 614.0(847.0,4 659.0) 7.1(3.3,18.0) 71.0(33.5,219.5) 17.0(11.0,30.6) P <0.001 <0.001 0.174 <0.001 ECOG评分 0~1(n=184) 642.5(418.5,1 367.3) 3.8(2.0,8.2) 58.6(26.1,172.0) 10.3(7.5,15.7) ≥2(n=16) 1 835.0(899.8,7 500.0) 14.8(2.9,35.6) 71.8(44.6,163.0) 16.5(12.2,26.3) P 0.001 0.011 0.494 0.015 结外受累个数 0~1(n=147) 565.0(395.5,1 136.0) 3.7(2.0,7.5) 51.8(25.8,160.0) 9.7(6.9,14.6) ≥2(n=53) 1 311.0(687.0,3 954.0) 5.0(2.4,18.2) 76.9(39.3,175.0) 14.6(10.2,26.0) P <0.001 0.023 0.207 <0.001 IPI评分 0~2(n=148) 511.0(394.5,1 058.8) 3.2(2.0,6.8) 49.3(24.9,142.6) 9.4(6.8,13.8) ≥3(n=52) 1 653.0(956.5,5 307.8) 9.9(3.8,19.8) 93.8(52.0,270.3) 17.4(11.2,30.6) P <0.001 <0.001 0.001 <0.001 DELa) 是(n=41) 674.0(358.0,2 015.0) 3.9(2.0,11.7) 40.5(20.8,94.0) 10.3(7.0,19.5) 否(n=158) 686.5(434.0,1 387.8) 3.8(2.0,9.2) 68.2(31.5,179.5) 9.4(6.8,13.8) P 0.873 0.695 0.047 0.131 Hans分型 GCB(n=87) 622.0(440.0,1 188.0) 3.4(2.0,6.0) 55.1(26.1,171.0) 10.1(6.9,14.6) non-GCB(n=113) 805.0(409.0,1 946.0) 4.8(2.2,12.8) 62.4(28.0,195.0) 10.5(8.1,19.7) P 0.180 0.013 0.433 0.189 注:a)1例患者是否为DEL未知。 表 2 2组患者治疗前血清细胞因子含量比较
M(P25,P75) 组别 IL-2R/(U·mL-1) IL-6/(pg·mL-1) IL-8/(pg·mL-1) TNF-α/(pg·mL-1) 复发组(n=45) 1 571.0(693.0,4 082.0) 7.1(2.6,17.6) 71.0(25.7,177.0) 14.7(10.2,29.4) 持续缓解组(n=155) 581.0(403.0,1 188.0) 3.5(2.0,7.8) 59.4(27.7,171.0) 9.9(7.0,15.0) P <0.001 0.010 0.630 <0.001 表 3 持续缓解组患者初发、缓解、持续缓解时血清细胞因子含量的比较
M(P25,P75) 细胞因子 治疗前 治疗6个周期开始前 持续缓解 IL-2R/(U·mL-1) 581.0(403.0,1 188.0) 452.0(356.0,588.0)1) 377.0(307.0,486.0)1)2) IL-6/(pg·mL-1) 3.5(2.0,7.8) 2.6(2.0,4.2)1) 2.0(2.0,2.6)1)2) IL-8/(pg·mL-1) 59.4(27.7,171.0) 20.5(9.7,47.8)1) 18.7(8.9,43.0)1) TNF-α/(pg·mL-1) 9.9(7.0,15.0) 8.1(6.5,10.4)1) 6.1(5.0,8.2)1)2) 与治疗前比较,1)P<0.05;与治疗6个周期开始前比较,2)P<0.05。 表 4 复发组患者初发、缓解、复发时血清细胞因子含量的比较
M(P25,P75) 细胞因子 治疗前 治疗6个周期开始前 复发期 IL-2R/(U·mL-1) 1 571.0(693.0,4 082.0) 491.0(385.5,646.0)1) 940.0(472.5,2 112.5)2) IL-6/(pg·mL-1) 7.1(2.6,17.6) 3.4(2.0,4.8)1) 6.4(4.4,10.8)2) IL-8/(pg·mL-1) 71.0(25.7,177.0) 25.2(14.7,53.9)1) 74.4(27.6,113.2)2) TNF-α/(pg·mL-1) 14.7(10.2,29.4) 7.4(5.7,8.9)1) 9.6(6.2,18.4)1) 与治疗前比较,1)P<0.05;与治疗6个周期开始前比较,2)P<0.05。 -
[1] 吴灿, 王辉, 张大川, 等. 高通量测序下弥漫性大B细胞淋巴瘤的研究[J]. 临床血液学杂志, 2021, 34(7): 523-526. doi: 10.13201/j.issn.1004-2806.2021.07.015 http://lcxz.cbpt.cnki.net/WKC/WebPublication/paperDigest.aspx?paperID=542cec9d-a50e-47ef-bd77-cdedb5daea3c
[2] 黄豪博, 黄晓玲, 范丽萍, 等. 治疗前血浆纤维蛋白原水平在弥漫大B细胞淋巴瘤患者预后判断中的价值[J]. 临床血液学杂志, 2022, 35(1): 58-62. http://lcxz.cbpt.cnki.net/WKC/WebPublication/paperDigest.aspx?paperID=6bab55a1-2648-43f1-b29d-4cdf74af10db
[3] Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia[J]. J Clin Oncol, 2005, 23(22): 5027-5033. doi: 10.1200/JCO.2005.09.137
[4] Propper DJ, Balkwill FR. Harnessing cytokines and chemokines for cancer therapy[J]. Nat Rev Clin Oncol, 2022, 19(4): 237-253. doi: 10.1038/s41571-021-00588-9
[5] Kadin ME. What Cytokines Can Tell Us About the Pathogenesis of Breast Implant-Associated Anaplastic Large Cell Lymphoma(BIA-ALCL)[J]. Aesthet Surg J, 2019, 39(Suppl_1): S28-S35.
[6] Tian S, Chen K, Xiao J, et al. Logistic regression models of cytokines in differentiating vitreoretinal lymphoma from uveitis[J]. J Clin Lab Anal, 2022, e24689.
[7] Yi JH, Yoon SE, Ryu KJ, et al. Pre-treatment serum IL-10 predicts the risk of secondary central nervous system involvement in patients with diffuse large B-cell lymphoma[J]. Cytokine, 2020, (129): 155048.
[8] Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms[J]. Blood, 2016, 127(20): 2375-2390. doi: 10.1182/blood-2016-01-643569
[9] Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma[J]. J Clin Oncol, 2007, 25(5): 579-586. doi: 10.1200/JCO.2006.09.2403
[10] Maurer MJ, Habermann TM, Shi Q, et al. Progression-free survival at 24 months(PFS24) and subsequent outcome for patients with diffuse large B-cell lymphoma(DLBCL)enrolled on randomized clinical trials[J]. Ann Oncol, 2018, 29(8): 1822-1827. doi: 10.1093/annonc/mdy203
[11] Yang Y, Wang Y, Liu X, et al. Progression-free survival at 24 months and subsequent survival of patients with extranodal NK/T-cell lymphoma: a China Lymphoma Collaborative Group(CLCG)study[J]. Leukemia, 2021, 35(6): 1671-1682. doi: 10.1038/s41375-020-01042-y
[12] Mao XC, Yang CC, Yang YF, et al. Peripheral cytokine levels as novel predictors of survival in cancer patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis[J]. Front Immunol, 2022, 13: 884592. doi: 10.3389/fimmu.2022.884592
[13] Zhang W, Gao Y, Hu G, et al. Lymphocyte may be a reference index of the outcome of cancer patients with COVID-19[J]. Aging(Albany NY), 2021, 13(6): 7733-7744.
[14] Conlon KC, Miljkovic MD, Waldmann TA. Cytokines in the Treatment of Cancer[J]. J Interferon Cytokine Res, 2019, 39(1): 6-21. doi: 10.1089/jir.2018.0019
[15] Hausmann JS. Targeting cytokines to treat autoinflammatory diseases[J]. Clin Immunol, 2019, 206: 23-32. doi: 10.1016/j.clim.2018.10.016
[16] Pernot B, Gyan E, Maillot F, et al. Lymphomas diagnosed in an internal medicine department compared to lymphomas diagnosed in other departments: Clinical and outcome differences[J]. Medicine(Baltimore), 2018, 97(47): e13228.
[17] 宋国丽, 王娅婕, 李增政, 等. 细胞因子与淋巴瘤关系的研究进展[J]. 白血病·淋巴瘤, 2020, 29(9): 556-569. doi: 10.3760/cma.j.cn115356-20200214-00042
[18] Stasi R, Zinzani L, Galieni P, et al. Clinical implications of cytokine and soluble receptor measurements in patients with newly-diagnosed aggressive non-Hodgkin's lymphoma[J]. Eur J Haematol, 1995, 54(1): 9-17.
[19] Sun F, Zhu J, Lu S, et al. An inflammation-based cumulative prognostic score system in patients with diffuse large B cell lymphoma in rituximab era[J]. BMC Cancer, 2018, 18(1): 5. doi: 10.1186/s12885-017-3931-z
[20] Li YL, Gu KS, Pan YY, et al. Peripheral blood lymphocyte/monocyte ratio at the time of first relapse predicts outcome for patients with relapsed or primary refractory diffuse large B-cell lymphoma[J]. BMC Cancer, 2014, 14: 341. doi: 10.1186/1471-2407-14-341
[21] Kim DY, Song MK, Chung JS, et al. Clinical impacts of inflammatory markers and clinical factors in patients with relapsed or refractory diffuse large B-cell lymphoma[J]. Blood Res, 2019, 54(4): 244-252. doi: 10.5045/br.2019.54.4.244
[22] Maeyama M, Sasayama T, Tanaka K, et al. Multi-marker algorithms based on CXCL13, IL-10, sIL-2 receptor, and beta2-microglobulin in cerebrospinal fluid to diagnose CNS lymphoma[J]. Cancer Med, 2020, 9(12): 4114-4125. doi: 10.1002/cam4.3048
[23] Dlouhy I, Filella X, Rovira J, et al. High serum levels of soluble interleukin-2 receptor(sIL2-R), interleukin-6(IL-6) and tumor necrosis factor alpha(TNF)are associated with adverse clinical features and predict poor outcome in diffuse large B-cell lymphoma[J]. Leuk Res, 2017, 59: 20-25. doi: 10.1016/j.leukres.2017.05.014
[24] Goto N, Tsurumi H, Goto H, et al. Serum soluble interleukin-2 receptor(sIL-2R)level is associated with the outcome of patients with diffuse large B cell lymphoma treated with R-CHOP regimens[J]. Ann Hematol, 2012, 91(5): 705-714.
[25] Lech-Maranda E, Bienvenu J, Broussais-Guillaumot F, et al. Plasma TNF-alpha and IL-10 level-based prognostic model predicts outcome of patients with diffuse large B-Cell lymphoma in different risk groups defined by the International Prognostic Index[J]. Arch Immunol Ther Exp(Warsz), 2010, 58(2): 131-141.
-




 下载:
下载: