Prognostic relevance of lymphocyte-to-monocyte ratio in decitabine and azacitidine in the treatment of myelodysplastic syndrome
-
摘要: 目的 探究淋巴细胞/单核细胞比值(LMR)、淋巴细胞亚群与去甲基化药物(HMA)治疗初发骨髓增生异常综合征(MDS)疗效的相关性。方法 收集徐州医科大学附属医院2017年1月—2022年2月收治的72例接受HMA单药治疗的MDS患者。根据首次入院时血常规结果计算得出LMR,并以中位数为临界值,将患者分为高LMR组(LMR≥4.80)36例和低LMR组(LMR < 4.80)36例;应用流式细胞术检测外周血淋巴细胞亚群(CD3+T细胞,CD4+T细胞及CD8+T细胞比例),分析LMR与治疗3个疗程后的治疗有效率(ORR)及中位总生存期(OS)的关系;淋巴细胞亚群在HMA治疗前后的变化以及与OS的相关性。结果 高LMR组与低LMR组治疗3个疗程后的中位生存期差异有统计学意义(P=0.036);多因素Cox回归分析显示3个疗程后LMR≥4.80是MDS患者OS的独立影响因素(P<0.05)。HMA治疗后CD8+T细胞比例显著升高(P=0.035)。结论 初发MDS患者LMR与HMA治疗MDS早期疗效及预后有关。患者首次入院的淋巴细胞亚群与HMA治疗MDS预后无明显相关性。
-
关键词:
- 淋巴细胞/单核细胞比值 /
- 骨髓增生异常综合征 /
- 去甲基化药物
Abstract: Objective To explore the correlation between lymphocyte-to-monocyte ratio(LMR), lymphocyte subsets and the efficacy of hypomethylating agent(HMA) in the treatment of primary myelodysplastic syndrome(MDS).Methods A total of 72 MDS patients who received HMA monotherapy admitted to the Affiliated Hospital of Xuzhou Medical University from January 2017 to February 2022 were collected. LMR was calculated according to the blood routine results at the first admission, and the median was the critical value, and the patients were divided into a high LMR group(LMR≥4.80) with 36 cases and a low LMR group(LMR < 4.80) with 36 cases. Cytometry was used to detect lymphocyte subsets in peripheral blood, and the relationship between LMR and related clinical indicators, overall response rate(ORR) after 3 courses of treatment, and median OS were analyzed.Results Multivariate Cox regression analysis showed that LMR≥4.80 after three courses of treatment was an independent factor for OS in MDS patients after three courses of demethylation drugs(P < 0.05), while the proportion of CD3+T cells, CD4+T cells and CD8+T cells was not independent factor for OS in MDS patients treated with HMAs. The proportion of CD8+T cells was increased after treatment of HMAs(P=0.035).Conclusion LMR in patients with newly diagnosed MDS is related to the early efficacy and prognosis of HMAs in the treatment of MDS. There was no significant correlation between lymphocyte subsets at first admission and the early efficacy of HMAs in the treatment of MDS. -

-
表 1 治疗前临床资料比较
例(%) 临床特征 总例数(n=72) 高LMR组(n=36) 低LMR组(n=36) P 性别 0.313 男 45(62.5) 21(58.3) 24(66.7) 女 27(37.5) 15(41.7) 12(33.3) 年龄/岁 0.105 < 60 24(33.3) 9(25.0) 15(41.7) ≥60 48(66.7) 27(75.0) 21(58.3) ANC/(×109·L-1) 0.002 < 0.8 31(43.1) 22(61.1) 9(25.0) ≥0.8 41(56.9) 14(38.9) 27(75.0) HGB/(g·L-1) 0.054 < 80 53(73.6) 23(69.3) 30(83.3) ≥80 19(26.4) 13(36.1) 6(16.7) ALC/(×109·L-1) 0.119 < 1.1 34(47.2) 20(55.6) 14(38.9) ≥1.1 38(52.8) 16(44.4) 22(61.1) AMC/(×109·L-1) 0.313 < 0.1 45(62.5) 21(58.3) 24(66.7) ≥0.1 27(37.5) 15(41.7) 12(33.3) PLT/(×109·L-1) 0.240 < 50 38(52.8) 17(47.2) 21(75.0) ≥50 34(47.2) 19(52.8) 15(25.0) 骨髓原始血细胞/% 0.116 < 5 42(58.3) 18(50.0) 24(66.7) ≥5 30(31.7) 18(50.0) 12(33.3) 细胞遗传学 0.524 极好/好 11(15.3) 7(19.4) 4(11.1) 中等 42(58.3) 21(58.3) 21(58.3) 差/极差 19(26.4) 8(22.2) 11(30.6) IPSS-R分级 0.477 低危 9(12.5) 4(11.1) 5(13.9) 中危 36(50.0) 16(44.4) 20(55.6) 高危/极高危 27(37.50) 16(44.4) 11(30.6) 药物 0.076 AZA 31(43.1) 19(52.8) 12(33.3) DAC 41(56.9) 17(47.2) 24(66.7) 表 2 治疗前2组血液学指标比较
X±S 临床指标 低LM组 高LMR组 t P ANC/(×109·L-1) 2.42±4.46 1.90±5.32 0.449 0.655 HGB/(g·L-1) 66.47±19.78 77.86±26.49 -2.066 0.042 PLT/(×109·L-1) 66.63±96.13 65.56±60.53 0.057 0.955 ALC/(×109·L-1) 1.56±2.52 1.48±1.97 0.166 0.868 AMC/(×109·L-1) 0.62±0.88 0.12±0.15 3.382 0.001 CD3+/% 70.55±12.66 73.84±13.83 -0.858 0.395 CD4+/% 43.09±9.86 42.65±10.68 0.145 0.886 CD8+/% 24.22±9.25 26.08±8.98 -0.704 0.485 表 3 治疗前与治疗3个疗程后临床指标变化
X±S 临床指标 治疗前 治疗后 P ANC/(×109·L-1) 2.15±4.88 1.64±4.00 0.293 HGB/(g·L-1) 72.17±23.91 77.18±30.43 0.168 PLT/(×109·L-1) 66.10±79.76 100.33±139.32 0.040 ALC/(×109·L-1) 1.53±2.25 1.27±1.31 0.375 AMC/(×109·L-1) 0.38±0.67 1.02±5.31 0.308 CD3+/% 72.14±13.72 72.15±17.78 0.992 CD4+/% 44.59±11.00 43.30±13.65 0.451 CD8+/% 23.38±9.50 24.19±9.63 0.035 表 4 2组患者治疗3个疗程后临床效果的比较
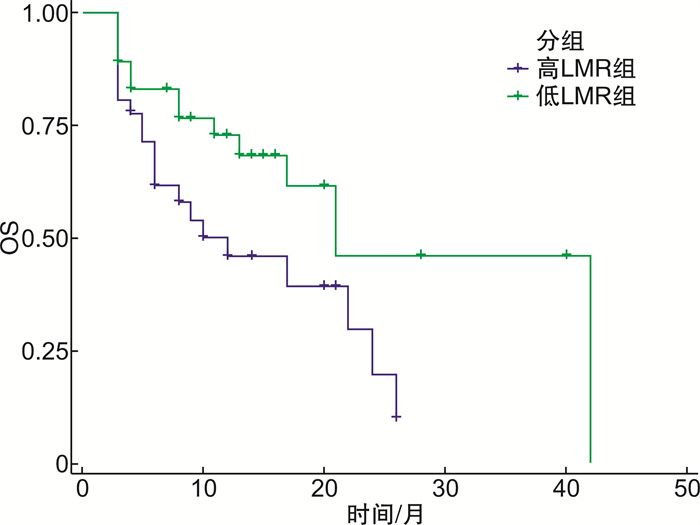
例 组别 例数 CR PR mCR HI SD PD ORR/% 低LMR组 36 3 5 7 4 8 9 52.8 高LMR组 36 0 4 3 2 7 20 25.0 χ2 5.844 P 0.014 表 5 2组患者生存情况比较
组别 中位生存期/月 OS/月 1年生存率/% 2年生存率/% 低LMR组 21 26 72.0 45.0 高LMR组 12 14 47.0 25.0 P 0.036 表 6 各因素对OS的影响
临床特征 单因素 多因素 HR(95%CI) P HR(95%CI) P 年龄>60岁 1.014(0.977~1.052) 0.466 性别 0.917(0.455~1.848) 0.809 ANC < 0.8×109/L 0.829(0.382~1.799) 0.630 HGB < 80 g/L 1.334(0.645~2.767) 0.443 PLT<50×109/L 0.270(0.093~0.787) 0.006 0.208(0.066~0.653) 0.007 CD3+ 1.017(0.988~1.047) 0.251 CD4+ 1.018(0.984~1.053) 0.311 CD8+ 1.019(0.981~1.057) 0.331 IPSS-R高/极高危 1.452(0.674~3.130) 0.328 骨髓原始细胞≥5% 0.598(0.300~1.193) 0.144 0.923(0.446~1.907) 0.828 LMR 2.063(1.011~4.210) 0.047 2.623(1.227~5.606) 0.013 -
[1] Nagel G, Weber D, Fromm E, et al. Epidemiological, genetic, and clinical characterization by age of newly diagnosed acute myeloid leukemia based on an academic population-based registry study(AMLSG BiO)[J]. Ann Hematol, 2017, 96(12): 1993-2003. doi: 10.1007/s00277-017-3150-3
[2] Nazha A, Sekeres MA, Bejar R, et al. Genomic Biomarkers to Predict Resistance to Hypomethylating Agents in Patients With Myelodysplastic Syndromes Using Artificial Intelligence[J]. JCO Precis Oncol, 2019, 3: PO. 19.00119.
[3] 曹蓝, 洪鸣. 骨髓增生异常综合征/骨髓增殖性肿瘤的治疗进展[J]. 临床血液学杂志, 2021, 34(9): 680-683. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXZ202109017.htm
[4] Shimono J, Izumiyama K, Ito S, et al. Lymphocyte-monocyte ratio(LMR)can predict bendamustine therapeutic efficacy in low-grade B-cell lymphoma[J]. Int J Lab Hematol, 2020, 42(4): 431-438. doi: 10.1111/ijlh.13216
[5] 王文儒, 杜宇, 许勇钢, 等. 骨髓增生异常综合征的临床特征及T淋巴细胞亚群分析[J]. 中华肿瘤防治杂志, 2022, 29(5): 329-336. https://www.cnki.com.cn/Article/CJFDTOTAL-QLZL202205004.htm
[6] Hu RJ, Ma JY, Hu G. Lymphocyte-to-monocyte ratio in pancreatic cancer: Prognostic significance and meta-analysis[J]. Clin Chim Acta, 2018, 481: 142-146. doi: 10.1016/j.cca.2018.03.008
[7] 徐卫峰, 关海旺, 潘靖南, 等. 不同年龄阶段淋巴细胞/单核细胞比值与稳定性冠心病Syntax积分的相关性研究[J]. 临床心血管病杂志, 2019, 35(7): 609-615. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXB201907005.htm
[8] Kahl BS, Yang DT. Follicular lymphoma: evolving therapeutic strategies[J]. Blood, 2016, 127(17): 2055-2063. doi: 10.1182/blood-2015-11-624288
[9] Du X, Lai YY, Xiao Z, et al. Efficacy, safety and pharmacokinetics of subcutaneous azacitidine in Chinese patients with higher risk myelodysplastic syndromes: Results from a multicenter, single-arm, open-label phase 2 study[J]. Asia Pac J Clin Oncol, 2018, 14(3): 270-278. doi: 10.1111/ajco.12835
[10] 邓理南. 170例骨髓增生异常综合征患者临床分析[D]. 武汉: 华中科技大学, 2019.
[11] Bewersdorf JP, Carraway H, Prebet T. Emerging treatment options for patients with high-risk myelodysplastic syndrome[J]. Ther Adv Hematol, 2020, 11: 2040620720955006.
[12] Bouchla A, Thomopoulos TP, Papageorgiou SG, et al. Predicting outcome in higher-risk myelodysplastic syndrome patients treated with azacitidine[J]. Epigenomics, 2021, 13(14): 1129-1143. doi: 10.2217/epi-2021-0124
[13] Liu T, Wang J, Li C, et al. Clinical effect of decitabine in the treatment of myelodysplastic syndrome and influencing factors[J]. Pak J Med Sci, 2020, 36(5): 1084-1088.
[14] Lübbert M, Suciu S, Hagemeijer A, et al. Decitabine improves progression-free survival in older high-risk MDS patients with multiple autosomal monosomies: results of a subgroup analysis of the randomized phase Ⅲ study 06011 of the EORTC Leukemia Cooperative Group and German MDS Study Group[J]. Ann Hematol, 2016, 95(2): 191-199. doi: 10.1007/s00277-015-2547-0
[15] Saeed L, Patnaik MM, Begna KH, et al. Prognostic relevance of lymphocytopenia, monocytopenia and lymphocyte-to-monocyte ratio in primary myelodysplastic syndromes: a single center experience in 889 patients[J]. Blood Cancer J, 2017, 7(3): e550. doi: 10.1038/bcj.2017.30
[16] Wang C, Yang Y, Gao S, et al. Immune dysregulation in myelodysplastic syndrome: Clinical features, pathogenesis and therapeutic strategies[J]. Crit Rev Oncol Hematol, 2018, 122: 123-132. doi: 10.1016/j.critrevonc.2017.12.013
[17] Helen LY, Bell E, Ettayebi I, et al. DNA hypomethylating agents increase activation and cytolytic activity of CD8+ T cells[J]. Mol Cell, 2021, 81(7): 1469-1483. e8. doi: 10.1016/j.molcel.2021.01.038
[18] Xiao N, He X, Niu H, et al. Increased Circulating CD4+ CXCR5+ Cells and IgG4 Levels in Patients with Myelodysplastic Syndrome with Autoimmune Diseases[J]. J Immunol Res, 2021, 2021: 4302515.
[19] Moskorz W, Cosmovici C, Jäger PS, et al. Myelodysplastic syndrome patients display alterations in their immune status reflected by increased PD-L1-expressing stem cells and highly dynamic exhausted T-cell frequencies[J]. Br J Haematol, 2021, 193(5): 941-945. doi: 10.1111/bjh.17461
[20] Badam TV, Hellberg S, Mehta RB, et al. CD4+ T-cell DNA methylation changes during pregnancy significantly correlate with disease-associated methylation changes in autoimmune diseases[J]. Epigenetics, 2021: 1-16.
[21] Li L, Yu S, Hu X, et al. Immunophenotypic changes of monocytes in myelodysplastic syndrome and clinical significance[J]. Clin Exp Med, 2022, Online ahead of print.
[22] Sarhan D, Wang J, Sunil Arvindam U, et al. Mesenchymal stromal cells shape the MDS microenvironment by inducing suppressive monocytes that dampen NK cell function[J]. JCI Insight, 2020, 5(5): e130155.
[23] Zhou T, Yin SJ, Wang P, et al. Association between TNF-α gene polymorphisms and susceptibility of myelodysplastic syndromes: a meta-analysis[J]. Hematology, 2021, 26(1): 1046-1056.
[24] Pollyea DA, Pratz K, Letai A, et al. Venetoclax with azacitidine or decitabine in patients with newly diagnosed acute myeloid leukemia: Long term follow-up from a phase 1b study[J]. Am J Hematol, 2021, 96(2): 208-217.
[25] Joji S, Koh I, Shinichi I, et al. Lymphocyte-monocyte ratio(LMR)can predict bendamustine therapeutic efficacy in low-grade B-cell lymphoma[J]. Int J Lab Hematol, 2020, 42(4): 431-438.
-




 下载:
下载: