Efficacy and safety analysis of allogeneic hematopoietic stem cell transplantation or tyrosine kinase inhibitors after CAR-T cell therapy for Philadelphia chromosome-positive acute B lymphoblastic leukemia
-
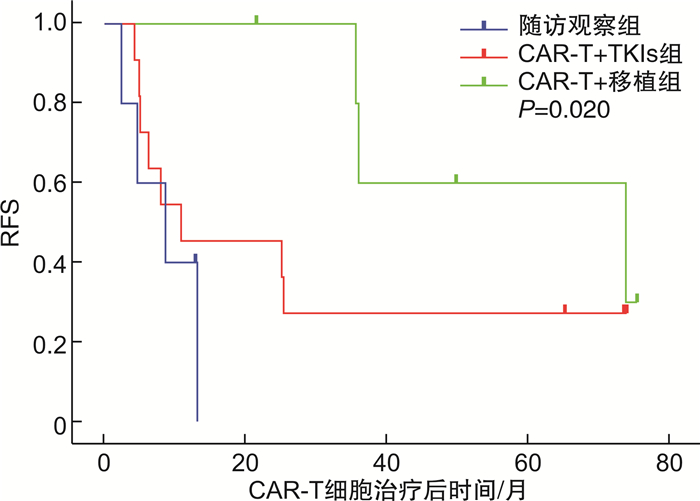
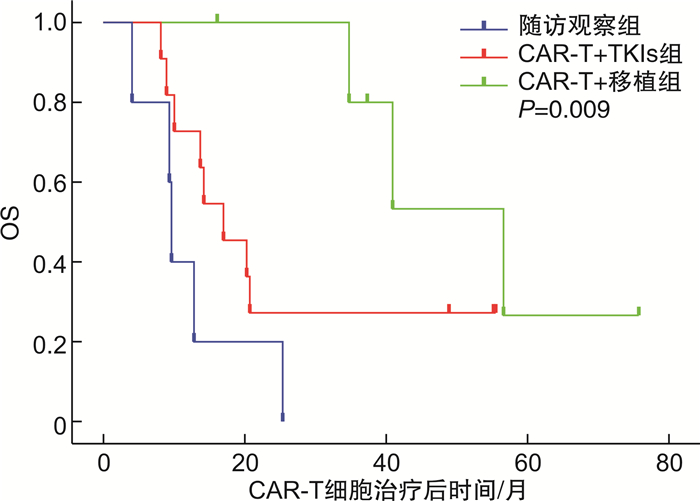
摘要: 目的 回顾性分析费城染色体阳性急性B淋巴细胞白血病(Ph+acute B-lymphocytic leukemia,Ph+B-ALL)患者行靶向CD19的嵌合抗原受体T细胞(chimeric antigen receptor T-cell,CAR-T)治疗达完全缓解后,采用随访观察、酪氨酸激酶抑制剂(tyrosine kinase inhibitors,TKIs)维持治疗或异基因造血干细胞移植(allogeneic hematopoietic stem cell transplantation,allo-HSCT)巩固治疗的疗效及安全性。方法 收集2017—2021年行自体CD19 CAR-T细胞治疗并获得完全缓解的Ph+B-ALL患者22例,根据CAR-T细胞治疗达完全缓解后治疗方式不同分为3组:随访观察组(5例),CAR-T+TKIs组(11例),CAR-T+移植组(6例),比较各组的总生存期、无复发生存期及不良反应。结果 22例患者中男女各11例,中位年龄34(7~66)岁。随访观察组、CAR-T+TKIs组、CAR-T+移植组的中位生存时间分别为9.7(95%CI 9.06~10.34)个月、17.1(95%CI 9.98~24.22)个月、56.8(95%CI 38.63~74.97)个月,1年无复发生存率分别为0、45.5%、100%。在安全性方面,移植后共4例出现急性移植物抗宿主病,1例出现慢性移植物抗宿主病,不良反应均可控,无移植相关死亡,CAR-T+TKIs组无严重的药物相关不良反应。结论 Ph+ALL行CAR-T细胞治疗后达完全缓解的患者,口服TKIs维持治疗或进行allo-HSCT可改善长期生存;与口服TKIs维持治疗相比,allo-HSCT能进一步降低早期复发风险。
-
关键词:
- 费城染色体 /
- 急性B淋巴细胞白血病 /
- 嵌合抗原受体T细胞 /
- 异基因造血干细胞移植 /
- 酪氨酸激酶抑制剂
Abstract: Objective To analyze the efficacy and safety of follow-up observation, tyrosine kinase inhibitors(TKIs), or allogeneic hematopoietic stem cell transplantation(allo-HSCT) in patients with Philadelphia chromosome-positive acute B lymphoblastic leukemia(Ph+B-ALL) who received CD19 chimeric antigen receptor T-cell(CAR-T) therapy to achieve complete remission(CR).Methods From 2017 to 2021, 22 patients with Ph+B-ALL who achieved CR after receiving autologous CD19 CAR-T cell therapy were divided into follow-up observation group(5 cases), CAR-T+TKIs group(11 cases) and CAR-T+HSCT group(6 cases). The overall survival, relapse-free survival and adverse events were compared among the groups.Results Among the 22 patients in this study, 11 cases were male and 11 cases were female, with a median age of 34(7-66) years old. The median overall survival in follow-up observation group, CAR-T+TKIs group and CAR-T+HSCT group were 9.7(95%CI 9.06-10.34) months, 17.1(95%CI 9.98-24.22) months, and 56.8(95%CI 38.63-74.97) months, respectively, and the 1-year relapse-free survival were 0, 45.5% and 100%, respectively. In terms of safety, 4 cases of acute graft-versus-host disease and 1 case of chronic graft-versus-host disease occurred after transplantation, and the adverse events were all controllable, with no transplant-related death. There were no serious drug-related adverse events in the CAR-T+TKIs group.Conclusion TKIs maintenance therapy or allo-HSCT improves long-term survival in Ph+B-ALL patients who achieve CR after CAR-T cell therapy. Compared to the maintenance therapy with TKIs, allo-HSCT provides a further reduction in the risk of early relapse. -

-
表 1 CAR-T细胞治疗前3组患者基线特征比较
基线特征 CAR-T+移植组(6例) CAR-T+TKIs组(11例) 随访观察组(5例) 统计量 P 年龄/岁 38(13.75,44) 31(20,49) 39(33,50) 0.584 0.567 性别/例(%) 4.248 0.153 男 5(83.3) 5(45.5) 1(20.0) 女 1(16.7) 6(54.5) 4(80.0) 复发次数/例(%) 1.086 0.737 < 2 3(50.0) 4(36.4) 1(20.0) ≥2或未缓解 3(50.0) 7(63.6) 4(80.0) CAR-T前移植史/例(%) 0 2(18.2) 1(20.0) 1.340 0.571 骨髓原始细胞比例/% 70.5(12.0,83.0) 60.3(38.6,74.8) 78.8(23.4,87.3) 1.545 0.462 髓外病变/例(%) 2(33.3) 2(18.2) 2(40.0) 1.256 0.569 -
[1] El Fakih R, Jabbour E, Ravandi F, et al. Current paradigms in the management of Philadelphia chromosome positive acute lymphoblastic leukemia in adults[J]. Am J Hematol, 2018, 93(2): 286-295. doi: 10.1002/ajh.24926
[2] 寇海明, 卢聪, 李成功, 等. 1例难治性原发中枢弥漫大B细胞淋巴瘤CAR-T细胞治疗中的多学科联合诊治[J]. 临床血液学杂志, 2022, 35(9): 621-625. doi: 10.13201/j.issn.1004-2806.2022.09.004
[3] 孙艳花, 孙艳丽, 崔景英, 等. 抗CD19嵌合抗原受体T细胞治疗复发难治B细胞非霍奇金淋巴瘤的单中心临床研究[J]. 临床血液学杂志, 2022, 35(1): 41-45. doi: 10.13201/j.issn.1004-2806.2022.01.008
[4] 茆诗源, 马瑞聪, 聂山林, 等. CART细胞治疗患者住院期间心血管不良事件发生的危险因素分析[J]. 临床心血管病杂志, 2021, 37(12): 1106-1111. doi: 10.13201/j.issn.1001-1439.2021.12.008
[5] 董斐斐, 傅维佳, 秦永文, 等. 嵌合抗原受体T细胞治疗的心血管毒性[J]. 临床心血管病杂志, 2020, 36(1): 83-85. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXB202001019.htm
[6] Pan J, Yang JF, Deng BP, et al. High efficacy and safety of low-dose CD19-directed CAR-T cell therapy in 51 refractory or relapsed B acute lymphoblastic leukemia patients[J]. Leukemia, 2017, 31(12): 2587-2593. doi: 10.1038/leu.2017.145
[7] Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia[J]. Sci Transl Med, 2014, 6(224): 224ra25.
[8] Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia[J]. N Engl J Med, 2014, 371(16): 1507-1517. doi: 10.1056/NEJMoa1407222
[9] Yang F, Yang X, Bao X, et al. Anti-CD19 chimeric antigen receptor T-cells induce durable remission in relapsed Philadelphia chromosome-positive ALL with T315I mutation[J]. Leuk Lymphoma, 2020, 61(2): 429-436. doi: 10.1080/10428194.2019.1663417
[10] Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial[J]. Lancet, 2015, 385(9967): 517-528. doi: 10.1016/S0140-6736(14)61403-3
[11] 何彩霞, 薛磊, 强萍, 等. CD19CAR-T细胞治疗复发难治Ph+急性B淋巴细胞白14例疗效及安全性[J]. 中华血液学杂志, 2020, 41(6): 490-494. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXZ202209013.htm
[12] Wang J, Hu Y, Huang H. Acute lymphoblastic leukemia relapse after CD19-targeted chimeric antigen receptor T cell therapy[J]. J Leukoc Biol, 2017, 102(6): 1347-1356. doi: 10.1189/jlb.5RU0817-315R
[13] Zhang LN, Song Y, Liu D. CD19 CAR-T cell therapy for relapsed/refractory acute lymphoblastic leukemia: factors affecting toxicities and long-term efficacies[J]. J Hematol Oncol, 2018, 11(1): 41. doi: 10.1186/s13045-018-0593-5
[14] Gauthier J, Bezerra ED, Hirayama AV, et al. Factors associated with outcomes after a second CD19-targeted CAR T-cell infusion for refractory B-cell malignancies[J]. Blood, 2021, 137(3): 323-335. doi: 10.1182/blood.2020006770
[15] Park JH, Rivière I, Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia[J]. N Engl J Med, 2018, 378(5): 449-459. doi: 10.1056/NEJMoa1709919
[16] Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia[J]. N Engl J Med, 2018, 378(5): 439-448. doi: 10.1056/NEJMoa1709866
[17] Hay KA, Gauthier J, Hirayama AV, et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy[J]. Blood, 2019, 133(15): 1652-1663. doi: 10.1182/blood-2018-11-883710
[18] Gu B, Shi BY, Zhang X, et al. Allogeneic haematopoietic stem cell transplantation improves outcome of adults with relapsed/refractory Philadelphia chromosome-positive acute lymphoblastic leukemia entering remission following CD19 chimeric antigen receptor T cells[J]. Bone Marrow Transplant, 2021, 56(1): 91-100.
[19] Jiang H, Li C, Yin P, et al. Anti-CD19 chimeric antigen receptor-modified T-cell therapy bridging to allogeneic hematopoietic stem cell transplantation for relapsed/refractory B-cell acute lymphoblastic leukemia: An open-label pragmatic clinical trial[J]. Am J Hematol, 2019, 94(10): 1113-1122.
[20] Zhang X, Lu XA, Yang J, et al. Efficacy and safety of anti-CD19 CAR T-cell therapy in 110 patients with B-cell acute lymphoblastic leukemia with high-risk features[J]. Blood Adv, 2020, 4(10): 2325-2338.
[21] Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond[J]. Blood, 2013, 122(4): 491-498.
[22] Sun Z, Yao B, Xie H, et al. Clinical Progress and Preclinical Insights Into Umbilical Cord Blood Transplantation Improvement[J]. Stem Cells Transl Med, 2022, 11(9): 912-926.
[23] Zhu X, Tang B, Sun Z. Umbilical cord blood transplantation: Still growing and improving[J]. Stem Cells Transl Med, 2021, 10(Suppl 2): S62-S74.
[24] Ruggeri A, Volt F, Locatelli F, et al. Unrelated Cord Blood Transplantation for Acute Leukemia Diagnosed in the First Year of Life: Outcomes and Risk Factor Analysis[J]. Biol Blood Marrow Transplant, 2017, 23(1): 96-102.
-




 下载:
下载: