-
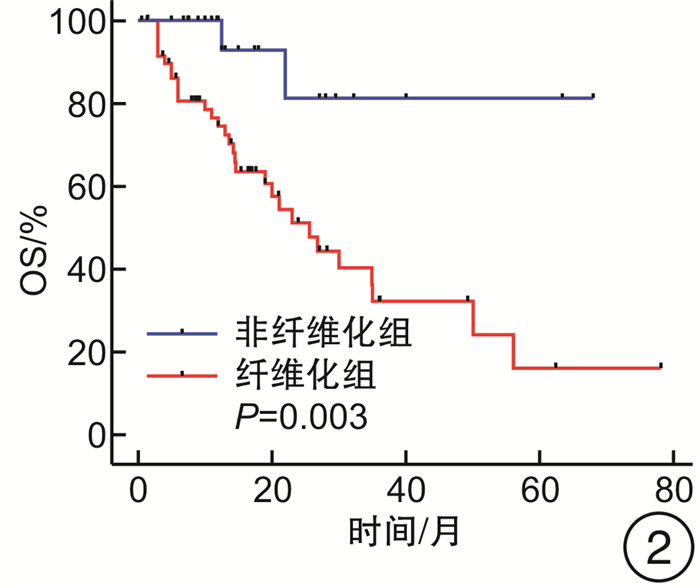
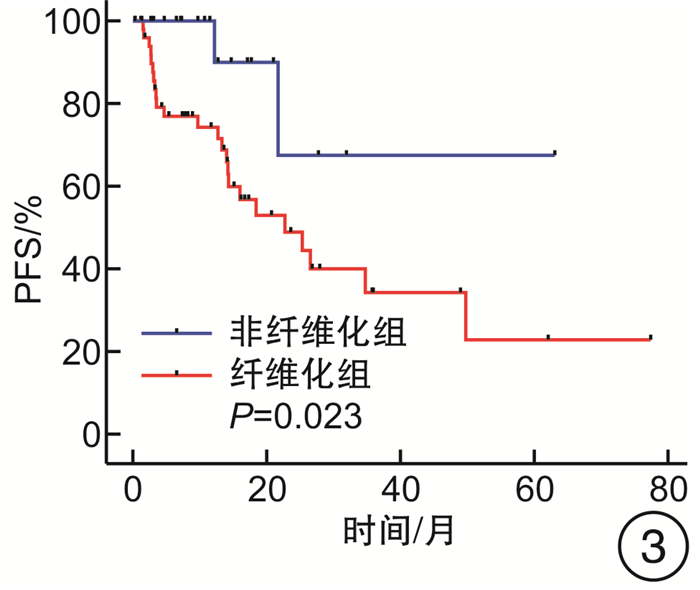
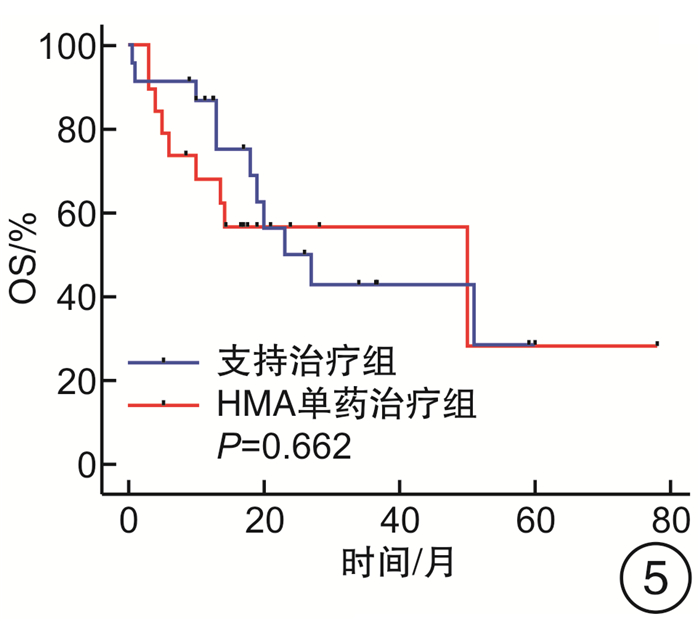
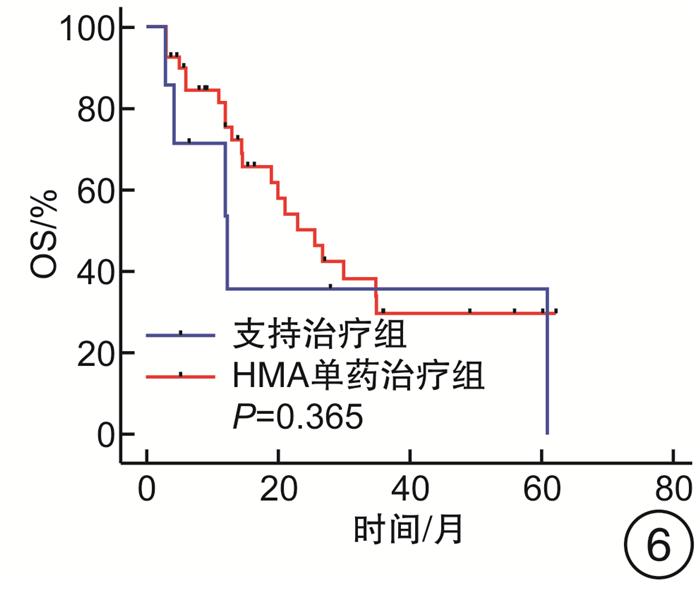
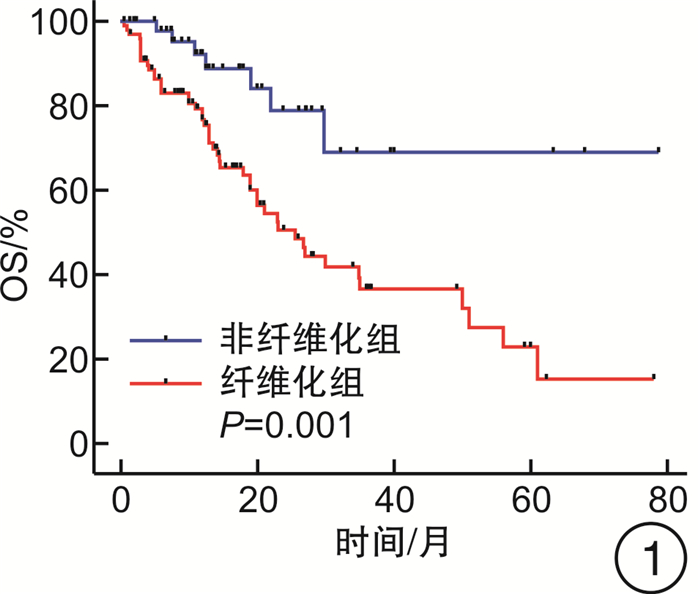
摘要: 目的 探究去甲基化药物(hypomethylating agent,HMA)在骨髓增生异常综合征(myelodysplastic syndrome,MDS)伴或不伴骨髓纤维化(MF)患者治疗中的临床效果。方法 收集山西白求恩医院2017年1月—2023年9月收治的147例MDS患者,其中MF 0级患者50例,MF 1~3级患者97例。根据骨髓纤维化的严重程度将接受HMA单药治疗的患者分为2组,非纤维化组(MF 0级)27例,纤维化期组(MF 1~3级)59例;根据是否接受HMA单药治疗将MF 1~3级患者分为2组,支持治疗组30例,HMA单药治疗组59例。分别比较2组患者的年龄、性别、血液学指标(白细胞计数、中性粒细胞计数、血红蛋白、血小板计数)、骨髓原始细胞比例、根据IPSS-R与IPSS-M的危险分层、WHO 2016分型、基因突变(TP53、ASXL1、U2AF1、DNMT3A、SF3B1、TET2、JAK2、RUNX1)以及治疗疗效。结果 非纤维化组与纤维化期组年龄、白细胞计数、中性粒细胞计数、血红蛋白、骨髓原始细胞比例、根据IPSS-R与IPSS-M的危险分层以及基因突变比较,差异无统计学意义(P>0.05),性别、血小板计数、中位总生存期(overall survival,OS)与中位无进展生存期(progression-free survival,PFS),差异有统计学意义(P < 0.05),对其中74例患者进行了疗效分析,差异无统计学意义(P>0.05);支持治疗组与HMA单药治疗组的年龄、性别、白细胞计数、中性粒细胞计数、血红蛋白以及中位OS、中位PFS比较,差异无统计学意义(P均>0.05),血小板计数、骨髓原始细胞比例、根据IPSS-R及IPSS-M的危险分层、WHO 2016分型、基因突变(ASXL1、TET2)差异有统计学意义(P < 0.05)。结论 MDS中骨髓纤维化与预后不良有关。接受HMA单药治疗并不能改善MDS伴骨髓纤维化患者的预后,并且接受HMA单药治疗并不能延长MDS伴骨髓纤维化患者的OS和PFS。Abstract: Objective To explore the clinical efficacy of hypomethylating agent(HMA) in the treatment of patients with or without myelofibrosis(MF) in myelodysplastic syndrome(MDS).Methods 147 MDS patients admitted to Shanxi Bethune Hospital from January 2017 to September 2023 were collected, including 50 patients with MF grade 0 and 97 patients with MF grade 1-3. Patients treated with HMA were divided into two groups according to the severity of myelofibrosis, 27 cases in the non-fibrotic group(MF 0) and 59 cases in the fibrotic phase group(MF 1-3); patients with MF grade 1-3 were divided into two groups according to whether they received HMA, 30 cases in the supportive treatment group and 59 cases in the HMA group. Age, sex, hematological parameters(white blood cell count, neutrophil count, hemoglobin, platelet count), proportion of bone marrow blasts, risk stratification according to IPSS-R and IPSS-M, WHO 2016 classification, gene mutations(TP53, ASXL1, U2AF1, DNMT3A, SF3B1, TET2, JAK2, RUNX1) and treatment efficacy were compared between the two groups.Results There were no significant differences in age, white blood cell count, neutrophil count, hemoglobin, percentage of bone marrow blasts, risk stratification according to IPSS-R versus IPSS-M, and gene mutations between non-fibrotic and fibrotic groups(P>0.05), sex, platelet count, median overall survival and median progression-free survival, statistically significant(P < 0.05), Seventy-four of these patients were analyzed for efficacy and there was no significant difference between subgroups(P>0.05); Supportive treatment vs HMA: There were no significant differences in age, sex, leukocyte count, neutrophil count, hemoglobin, and median overall survival versus median progression-free survival(P>0.05). Platelet count, proportion of bone marrow blasts, risk stratification according to IPSS-R and IPSS-M, WHO 2016 typing, and gene mutations(ASXL1, TET2) were statistically significant(P < 0.05).Conclusion Myelofibrosis in MDS is associated with poor prognosis. Treatment with HMA alone did not improve outcomes in patients with MDS and myelofibrosis, and treatment with HMA alone did not prolong overall survival and progression-free survival in patients with MDS and myelofibrosis.
-
Key words:
- hypomethylating agent /
- myelodysplastic syndrome /
- myelofibrosis
-

-
表 1 接受HMA单药治疗的非纤维化组与纤维化组的临床和实验室数据
类型 非纤维化组(27例) 纤维化组(59例) P 年龄/岁 64(46~78) 64(25~81) 0.881 性别/例(%) 0.049 男 23(85.19) 38(64.41) 女 4(14.81) 21(35.59) 白细胞计数/(×109/L) 1.78(0.30~16.60) 2.40(0.10~205.60) 0.256 中性粒细胞计数/(×109/L) 0.60(0.10~13.65) 1.06(0.06~196.14) 0.216 血红蛋白/(g/L) 77(30~127) 71(25~135) 0.216 血小板计数/(×109/L) 51(6~640) 45(1~252) 0.049 原始细胞计数/% 6.0(0.5~19.0) 5.0(0~20.0) 0.793 IPSS-R/例(%) 0.851 极低危 0 1(1.69) 低危 3(11.11) 11(18.64) 中危 8(29.63) 15(25.42) 高危 11(40.74) 23(38.98) 极高危 5(18.52) 9(15.25) IPSS-M/例(%) 0.098 极低危 1(3.70) 13(22.03) 低危 4(14.81) 3(5.08) 中等偏低危 1(3.70) 1(1.69) 中等偏高危 4(14.81) 13(22.03) 高危 11(40.74) 13(22.03) 极高危 6(22.22) 16(27.12) WHO 2016分型/例(%) 0.635 MDS-SLD 1(3.70) 1(1.69) MDS-MLD 5(18.52) 12(20.34) MDS-RS-SLD 1(3.70) 2(3.39) MDS-RS-MLD 0 4(6.78) MDS-del(5q) 1(3.70) 2(3.39) MDS-EB-1 9(33.33) 20(33.90) MDS-EB-2 10(37.04) 14(23.73) MDS-U 0 4(6.78) 基因突变/例(%) TP53 4(14.81) 12(20.34) 0.541 ASXL1 9(33.33) 14(23.73) 0.350 U2AF1 5(18.53) 9(15.25) 0.704 DNMT3A 2(7.41) 8(13.56) 0.409 SF3B1 3(11.11) 7(11.86) 0.919 TET2 7(25.93) 7(11.86) 0.101 JAK2 1(3.70) 6(10.17) 0.309 RUNX1 8(29.63) 8(13.56) 0.076 疗效评估(n=74)/例(%) 24(32.43) 50(67.57) 0.140 CR/PR/SD 19(79.17) 31(62.00) PD 5(20.83) 19(38.00) 表 2 支持治疗组与HMA单药治疗组的临床和实验室数据
类型 支持治疗组(30例) HMA单药治疗组(59例) P 年龄/岁 60.5(23.0~88.0) 64.0(25.0~81.0) 0.284 性别/例(%) 0.832 男 20(66.67) 28(64.41) 女 10(33.33) 21(35.59) 白细胞计数/(×109/L) 2.8(0.9~94.9) 2.4(0.1~205.6) 0.825 中性粒细胞计数/(×109/L) 1.50(0.07~88.64) 1.06(0.06~196.14) 0.860 血红蛋白/(g/L) 68.5(34.0~135.4) 71.0(25.0~135.0) 0.916 血小板计数/(×109/L)
69(6~129 3)
45(1~252)0.015 原始细胞计数/% 1.2(0.5~10.5) 5.0(0~20) < 0.001 IPSS-R/例(%) 0.003 极低危 1(3.33) 1(1.69) 低危 12(40.00) 11(18.64) 中危 14(46.67) 15(25.42) 高危 2(6.67) 23(38.98) 极高危 1(3.33) 9(15.25) IPSS-M/例(%) < 0.001 极低危 2(6.67) 13(22.03) 低危 9(30.00) 3(5.08) 中等偏低危 10(33.33) 1(1.69) 中等偏高危 3(10.00) 13(22.03) 高危 3(10.00) 13(22.03) 极高危 3(10.00) 16(27.12) WHO 2016分型/例(%) 0.005 MDS-SLD 0 1(1.69) MDS-MLD 14(46.67) 12(20.34) MDS-RS-SLD 2(6.67) 2(3.39) MDS-RS-MLD 5(16.67) 4(6.78) MDS-del(5q) 2(6.67) 2(3.39) MDS-EB-1 1(3.33) 20(33.90) MDS-EB-2 2(6.67) 14(23.73) MDS-U 4(13.33) 4(6.78) 基因突变/例(%) TP53 3(10.00) 12(20.34) 0.218 ASXL1 1(3.33) 14(23.73) 0.015 U2AF1 5(16.67) 9(15.25) 0.863 DNMT3A 3(10.00) 8(13.56) 0.630 SF3B1 4(13.33) 7(11.86) 0.842 TET2 9(30.00) 7(11.86) 0.035 JAK2 1(3.33) 6(10.17) 0.257 RUNX1 2(6.67) 8(13.56) 0.330 -
[1] Gangat N, Patnaik MM, Begna K, et al. Primary Myelodysplastic Syndromes: The Mayo Clinic Experience With 1000 Patients[J]. Mayo Clin Proc, 2015, 90(12): 1623-1638. doi: 10.1016/j.mayocp.2015.08.022
[2] STeensma DP. Myelodysplastic syndromes current treatment algorithm 2018[J]. Blood Cancer J, 2018, 8(5): 47. doi: 10.1038/s41408-018-0085-4
[3] Fu B, Jaso JM, Sargent RL, et al. Bone marrow fibrosis in patients with primary myelodysplastic syndromes has prognostic value using current therapies and new risk stratification systems[J]. Mod Pathol, 2014, 27(5): 681-689. doi: 10.1038/modpathol.2013.187
[4] Ramos F, Robledo C, Izquierdo-garcía FM, et al. Bone marrow fibrosis in myelodysplastic syndromes: a prospective evaluation including mutational analysis[J]. Oncotarget, 2016, 7(21): 30492-30503. doi: 10.18632/oncotarget.9026
[5] Khan M, Muzzafar T, Kantarjian H, et al. Association of bone marrow fibrosis with inferior survival outcomes in chronic myelomonocytic leukemia[J]. Ann Hematol, 2018, 97(7): 1183-1191. doi: 10.1007/s00277-018-3289-6
[6] Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia[J]. Blood, 2016, 127(20): 2391-2405. doi: 10.1182/blood-2016-03-643544
[7] Khoury JD, Solary E, Abla O, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms[J]. Leukemia, 2022, 36(7): 1703-1719. doi: 10.1038/s41375-022-01613-1
[8] Ebaid A, Cingam SY, Boyce T, et al. Comparative Analysis for Effectiveness of Azacitidine Versus Decitabine in Acute Myeloid Leukemia and Myelodysplastic Syndromes with Marrow Fibrosis: A Single Institution Experience[J]. Blood, 2019, 134(Supplement_1): 5099. doi: 10.1182/blood-2019-131881
[9] Groarke EM, Maung SW, Ewins K, et al. The Role of Marrow Fibrosis in the Prognosis and Treatment of Myelodysplastic Syndromes: a Single Center Retrospective Study[J]. Blood, 2016, 128(22): 5524. doi: 10.1182/blood.V128.22.5524.5524
[10] Hammond D, Jamali M, Wells RA, et al. Impact of Bone Marrow Fibrosis in MDS Patients Treated with Azacitidine[J]. Blood, 2016, 128(22): 4339. doi: 10.1182/blood.V128.22.4339.4339
[11] Reda G, Riva M, Fattizzo B, et al. Bone Marrow Fibrosis and Early Hematological Response as Predictors of Poor Outcome in Azacitidine Treated High Risk-Patients With Myelodysplastic Syndromes or Acute Myeloid Leukemia[J]. Semin Hematol, 2018, 55(4): 202-208. doi: 10.1053/j.seminhematol.2018.02.005
[12] Shahidi R, Mohamed M, Sharma A, et al. Bone marrow fibrosis impact on response to azacitidine in myelodysplastic syndromes[J]. Pathology, 2022, 54(6): 763-767. doi: 10.1016/j.pathol.2022.02.011
[13] Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group(IWG)response criteria in myelodysplasia[J]. Blood, 2006, 108(2): 419-425. doi: 10.1182/blood-2005-10-4149
[14] Hoang NM, Rui L. DNA methyltransferases in hematological malignancies[J]. J Genet Genomics, 2020, 47(7): 361-372. doi: 10.1016/j.jgg.2020.04.006
[15] Imataki O, Ishida T, Kubo H, et al. A Case of Tyrosine Kinase Inhibitor-Resistant Chronic Myeloid Leukemia, Chronic Phase with ASXL1 Mutation[J]. Case Rep Oncol, 2020, 13(1): 449-455. doi: 10.1159/000506452
[16] Dolgikh TY, Senchukova SR, Vinogradova EV, et al. Specific Features of Interactions between Megakaryocytic and Granulocytic Hematopoiesis Lineages and Myelofibrosis during the Acute Phase of Chronic Myeloid Leukemia, Chronic Lymphocytic Leukemia, and Multiple Myeloma[J]. Bull Exp Biol Med, 2020, 168(6): 734-738. doi: 10.1007/s10517-020-04791-z
[17] Dolgikh TY, Domnikova NP, Tornuev YV, et al. Incidence of Myelofibrosis in Chronic Myeloid Leukemia, Multiple Myeloma, and Chronic Lymphoid Leukemia during Various Phases of Diseases[J]. Bull Exp Biol Med, 2017, 162(4): 483-487. doi: 10.1007/s10517-017-3645-x
[18] Park SJ, Lee HW, Jeong SH, et al. Acquisition of a BCR-ABL1 transcript in a patient with disease progression from MDS with fibrosis to AML with myelodysplasia-related changes[J]. Ann Clin Lab Sci, 2011, 41(4): 379-384.
[19] Zhang X, Wang F, Yu J, et al. Significance of bone marrow fibrosis in acute myeloid leukemia for survival in the real-world[J]. Front Oncol, 2022, 12: 971082. doi: 10.3389/fonc.2022.971082
[20] Sokol K, Tremblay D, Bhalla S, et al. Implications of Mutation Profiling in Myeloid Malignancies-PART 2: Myeloproliferative Neoplasms and Other Myeloid Malignancies[J]. Oncology(Williston Park), 2018, 32(5): e45-e51.
[21] Craver BM, Elalaoui K, Scherber RM, et al. The Critical Role of Inflammation in the Pathogenesis and Progression of Myeloid Malignancies[J]. Cancers(Basel), 2018, 10(4): 104.
[22] Chifotides HT, Verstovsek S, Bose P. Association of Myelofibrosis Phenotypes with Clinical Manifestations, Molecular Profiles, and Treatments[J]. Cancers(Basel), 2023, 15(13): 3331.
[23] Tefferi A, Lasho TL, Patnaik MM, et al. Targeted next-generation sequencing in myelodysplastic syndromes and prognostic interaction between mutations and IPSS-R[J]. Am J Hematol, 2017, 92(12): 1311-1317. doi: 10.1002/ajh.24901
-

| 引用本文: | 祝坤, 张婕, 贡蓉. 去甲基化药物对骨髓增生异常综合征伴骨髓纤维化患者的疗效分析[J]. 临床血液学杂志, 2024, 37(5): 333-338. doi: 10.13201/j.issn.1004-2806.2024.05.009 |
| Citation: | ZHU Kun, ZHANG Jie, GONG Rong. Effect of hypomethylating agent on myelodysplastic syndrome with myelofibrosis[J]. J Clin Hematol, 2024, 37(5): 333-338. doi: 10.13201/j.issn.1004-2806.2024.05.009 |
- Figure 1.
- Figure 2.
- Figure 3.
- Figure 4.
- Figure 5.
- Figure 6.
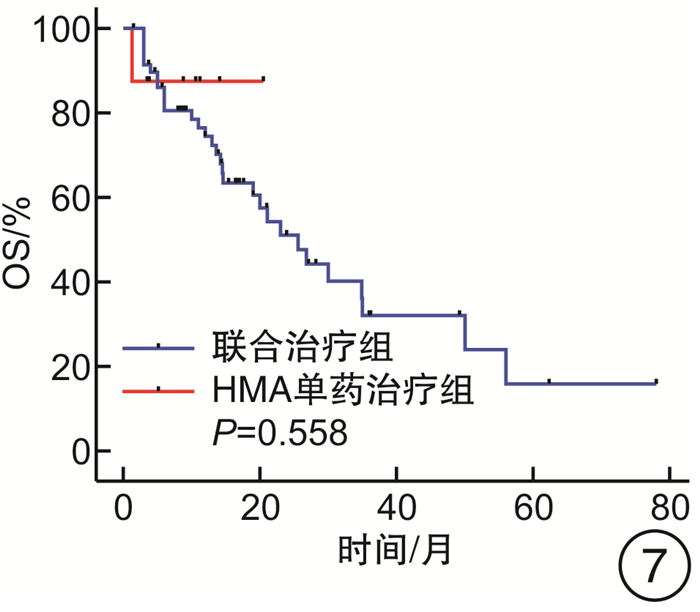
- Figure 7.



 下载:
下载: