Comparison of frontline immunosuppressive therapy and hematopoietic stem cell transplantation for severe hepatitis-associated aplastic anemia
-
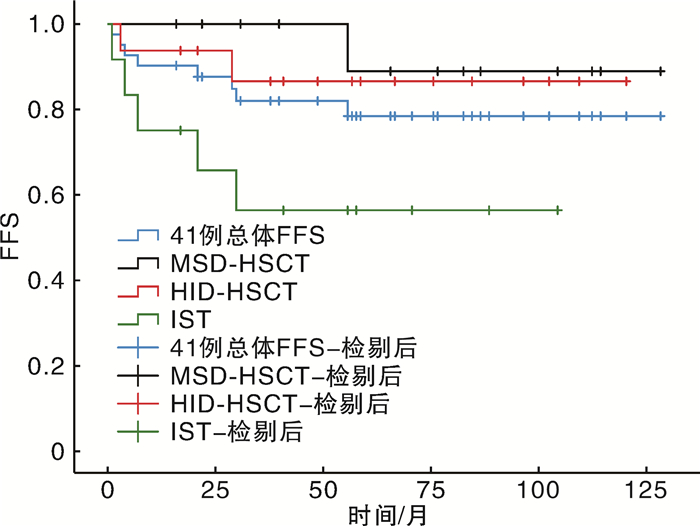
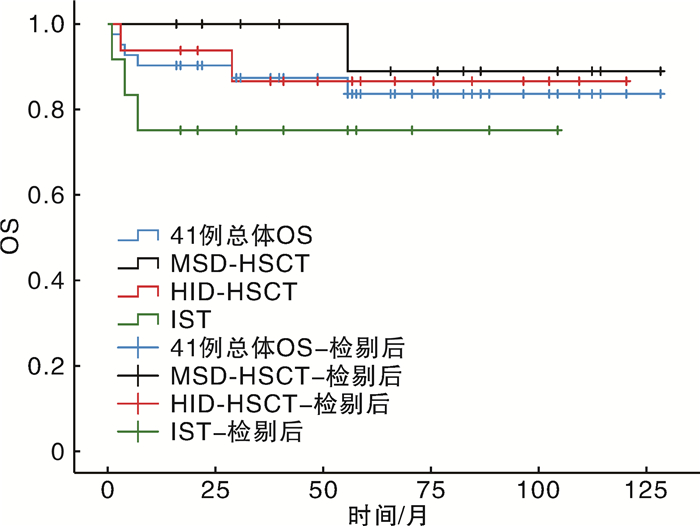
摘要: 目的 比较一线免疫抑制治疗(immunosuppressive therapy,IST)及造血干细胞移植(hematopoietic stem cell transplantation,HSCT)治疗肝炎相关性再生障碍性贫血(hepatitis-associated aplastic anemia,HAAA)的疗效。方法 选择2013年1月1日至2022年8月31日诊断为重度或极重度HAAA的患者41例,进行一线IST(12例)或HSCT治疗(29例),其中同胞全相合供者异基因造血干细胞移植(matched-sibling donor hematopoietic stem cell transplantation,MSD-HSCT)13例,单倍体异基因造血干细胞移植(haplo-identical donor hematopoietic stem cell transplantation,HID-HSCT)16例。结果 除死亡事件外,所有患者移植后中位随访时间为67(16~129)个月。MSD-HSCT组和HID-HSCT组患者造血重建时间、感染发生率、Ⅱ~Ⅳ度急性移植物抗宿主病、慢性移植物抗宿主病发生率差异均无统计学意义。IST组治疗失败率明显高于HSCT组(41.7% vs 10.3%,P=0.034)。整个队列显示5年总生存率(overall survival,OS)和无失败生存率(failure-free survival,FFS)分别为85.4%和80.5%。MSD-HSCT组、HID-HSCT组、IST组5年OS率分别为92.3%、87.5%和75.0%,5年FFS率分别为92.3%、87.5%和58.3%。HSCT组和IST组5年OS率差异无统计学意义;MSD-HSCT组5年FFS率较IST组明显延长(P=0.039);MSD-HSCT组和HID-HSCT组5年OS率及FFS率差异均无统计学意义。结论 HSCT较IST更适合于极重型或爆发性HAAA患者。MSD-HSCT仍是HAAA治疗的一线选择,对于没有MSD供者的年轻患者来说,HID-HSCT可以替代IST作为一线选择。
-
关键词:
- 肝炎相关再生障碍性贫血 /
- 异基因造血干细胞移植 /
- 免疫抑制治疗
Abstract: Objective To compare the clinical efficacy between frontline immunosuppressive therapy(IST) and hematopoietic stem cell transplantation(HSCT) for patients with severe hepatitis-associated aplastic anemia(HAAA).Methods A total of 41 patients who received frontline IST(12 cases) or HSCT(29 cases) between January 1, 2013 and August 31, 2022 were retrospectively analyzed. Among 29 patients undergoing HSCT, there were 13 cases of matched-sibling donor hematopoietic stem cell transplantation(MSD-HSCT) and 16 cases of haplo-identical donor hematopoietic stem cell transplantation(HID-HSCT).Results Except for death events, the median follow-up time for all patients after transplantation was 67(16-129) months. There was no significant difference in hematopoietic reconstruction time, the infection rate, the incidences of gradeⅡ-Ⅳ acute graft-versus-host disease and chronic graft-versus-host disease between the MSD-HSCT group and the HID-HSCT group. The incidence of treatment failure in the IST group was higher than that in the HSCT group(41.7% vs 10.3%, P=0.034). The estimated 5-year overall survival(OS) and failure-free survival(FFS) were 85.4% and 80.5% for all of the 41 patients. The estimated 5-year OS in the MSD-HSCT group, HID-HSCT group and IST group were 92.3%, 87.5% and 75.0%, respectively. The estimated 5-year FFS in the MSD-HSCT group, HID-HSCT group and IST group were 92.3%, 87.5% and 58.3%, respectively. There was no significant difference in the estimated 5-year OS between the HSCT group and the IST group, but the estimated 5-year FFS in the MSD-HSCT group was significantly higher than that in the IST group(P=0.039). There was no significant difference in the expected 5-year OS and FFS between the MSD-HSCT group and the HID-HSCT group.Conclusion HSCT is more suitable for patients with severe HAAA than IST. MSD-HSCT remains the first-line option for HAAA, and HID-HSCT can replace IST as the first-line option for younger patients without a MSD donor. The frontline haplo-HSCT was an effective and safe approach for the treatment of patients with SAA who lack a HLA-matched sibling donor. -

-
表 1 IST组和HSCT组患者基本临床资料比较
项目 IST组
(12例)HSCT组
(29例)P 年龄/岁 24(11~57) 26(19~43) 0.58 男∶女/例 8∶4 18∶11 0.54 ECOG评分0~1/例(%) 9(75.0) 23(79.3) 0.53 肝炎到AA诊断时间/月 4(2~7) 4(1.5~6) 0.41 AA诊断至治疗时间/d 7(1~14) 31(16~57) 0.39 治疗前感染/例(%) 5(41.7) 22(75.9) 0.04 肝功能(峰值) 谷丙转氨酶/(U/L) 1 432±692 1 687±812 0.45 谷草转氨酶/(U/L) 1 038±609 986±589 0.38 总胆红素/(μmol/L) 168±79 171±91 0.21 疾病状态/例(%) < 0.01 SAA 10(83.3) 3(10.3) VSAA 2(16.7) 26(89.7) 表 2 MSD-HSCT组和HID-HSCT组患者的基本临床资料及疗效
项目 MSD-HSCT
(13例)HID-HSCT
(16例)P 年龄/岁 27(21~43) 25(19~39) 0.41 男∶女/例 8∶5 10∶6 0.63 HCT-CT评分0~1分/例(%) 12(92.3) 14(87.5) 0.58 疾病状态/例(%) 0.42 SAA 2(15.4) 1(6.2) VSAA 11(84.6) 15(93.8) 确诊至移植时间/d 29(16~41) 24(19~57) 0.37 供-受者性别匹配/例(%) 0.39 男-男 5(38.5) 6(37.5) 男-女 3(23.1) 3(18.8) 女-女 2(15.4) 3(18.8) 女-男 3(23.1) 4(25.0) 预处理方案/例(%) 0.43 Flu/Cy+ATG 7(53.8) 7(43.8) Bu/Cy+ATG 6(46.2) 9(56.2) 造血植入时间/d 中性粒细胞植入 13(10~17) 14(10~18) 0.38 血小板植入 14(9~21) 15(11~19) 0.30 移植后1个月完全植入/例(%) 13(100.0) 16(100.0) 原发病植入失败/例(%) 0 0 Ⅱ~Ⅳ度急性GVHD/例(%) 6(46.2) 10(62.5) 0.31 慢性GVHD/例(%) 1(7.7) 3(18.8) 0.38 感染/例(%) 细菌/真菌 9(69.2) 14(87.5) 0.23 CMV 5(38.5) 11(68.8) 0.63 EBV 3(23.1) 5(31.3) 0.51 PTLD/例(%) 1(7.7) 2(12.5) 0.58 死亡/例(%) 1(7.7) 2(12.5) 0.58 感染 0 1(6.3) GVHD 0 1(6.3) 其他 1(7.7) 0 -
[1] 尤亚红, 孟宪彬, 李星鑫, 等. 肝炎相关再生障碍性贫血患者长期随访研究[J]. 中国实验血液学杂志, 2017, 25(4): 1130-1135. https://www.cnki.com.cn/Article/CJFDTOTAL-XYSY201704035.htm
[2] Yoshida N, Kobayashi R, Yabe H, et al. First-line treatment for severe aplastic anemia in children: bone marrow transplantation from, a matched family donor versus immunosuppressive therapy[J]. Haematologica, 2014, 99(12): 1784-1791. doi: 10.3324/haematol.2014.109355
[3] Xu LP, Xu ZL, Wang SQ, et al. Long-term follow-up of haploidentical transplantation in relapsed/refractory severe aplastic anemia: a multicenter prospective study[J]. Sci Bull(Beijing), 2022, 67(9): 963-970.
[4] 张之南, 沈悌. 血液病诊断及疗效标准[M]. 3版. 北京: 科学出版社, 2007: 19-23.
[5] 肖方, 刘强, 范丹, 等. 一线与挽救性单倍体造血干细胞移植治疗重型再生障碍性贫血患者的疗效比较[J]. 中国实验血液学杂志, 2020, 28(5): 1683-1688. https://www.cnki.com.cn/Article/CJFDTOTAL-XYSY202005047.htm
[6] Zhang X, Yang W, Yang D, et al. Comparison of hematopoietic stem cell transplantation and immunosuppressive therapy as the first-line treatment option for patients with severe hepatitis associated aplastic anemia[J]. Front Immunol, 2023, 14: 1146997. doi: 10.3389/fimmu.2023.1146997
[7] Gafar F, Arifin H, Jurnalis YD, et al. Antituberculosis drug induced liver injury in children: incidence and risk factors during the two-month intensive phase of therapy[J]. Pediatr Infect Dis J, 2019, 38: 50-53. doi: 10.1097/INF.0000000000002192
[8] Takehiko M, Yasushi O, Yukiyasu O, et al. Outcome of allogeneic hematopoietic stem cell transplantation in adult patients with hepatitis associated aplastic anemia[J]. Int J Hematol, 2019, 109(6): 711-717. doi: 10.1007/s12185-019-02644-8
[9] Xu ZL, Zhou M, Jia JS, et al. Immunosuppressive therapy versus haploidentical transplantation in adults with acquired severe aplastic anemia[J]. Bone Marrow Transplant, 2019, 54(8): 1319-1326. doi: 10.1038/s41409-018-0410-3
[10] Zhang Y, Huo J, Liu L, et al. Comparison of hematopoietic stem cell transplantation outcomes using matched sibling donors, haploidentical donors, and immunosuppressive therapy for patients with acquired aplastic anemia[J]. Front Immunol, 2022, 13: 837335. doi: 10.3389/fimmu.2022.837335
[11] 李瑞鑫, 金媛媛, 杨岩, 等. 艾曲泊帕联合强化免疫抑制疗法治疗成人重型再生障碍性贫血疗效的预测因素[J]. 临床血液学杂志, 2022, 35(5): 333-337. https://lcxy.whuhzzs.com/article/doi/10.13201/j.issn.1004-2806.2022.05.007
[12] Yang W, Zhao X, Liu X, et al. Hetrombopag plus porcine ATG and cyclosporine for the treatment of aplastic anaemia: Early outcomes of a prospective pilot study[J]. Exp Hematol Oncol, 2023, 12(1): 16. doi: 10.1186/s40164-023-00377-3
[13] 宫跃敏, 马永超, 陈小玉, 等. 抗人T细胞猪免疫球蛋白联合艾曲泊帕治疗初发重型再生障碍性贫血疗效分析[J]. 临床血液学杂志, 2023, 36(7): 482-487. https://lcxy.whuhzzs.com/article/doi/10.13201/j.issn.1004-2806.2023.07.006
[14] Scheinberg P. Acquired severe aplastic anaemia: How medical therapy evolved in the 20th and 21st centuries[J]. Br J Haematol, 2021, 194(6): 954-969. doi: 10.1111/bjh.17403
[15] Babushok DV, Grignon AL, Li Y, et al. Disrupted lymphocyte homeostasis in hepatitis-associated acquired aplastic anemia is associated with short telomeres[J]. Am J Hematol, 2016, 91(2): 243-247. doi: 10.1002/ajh.24256
[16] Mohseny AB, Eikema DA, Neven B, Kröger N, et al. Hematopoietic stem cell transplantation for hepatitis-associated aplastic anemia following liver transplantation for nonviral hepatitis: A retrospective analysis and a review of the literature by the severe aplastic anemia working party of the European society for blood and marrow transplantation[J]. J Pediatr Hematol Oncol, 2021, 43(7): e1025-e1029. doi: 10.1097/MPH.0000000000001991
[17] Mori T, Onishi Y, Ozawa Y, et al. Outcome of allogeneic hematopoietic stem cell transplantation in adult patients with hepatitisassociated aplastic anemia[J]. Int J Hematol, 2019, 109(6): 711-717. doi: 10.1007/s12185-019-02644-8
[18] 杜志丛, 赵艳丽, 曹星玉, 等. 单倍型供者造血干细胞移植治疗重型再生障碍性贫血的单中心研究[J]. 临床血液学杂志, 2023, 36(5): 366-372. https://lcxy.whuhzzs.com/article/doi/10.13201/j.issn.1004-2806.2023.05.013
[19] Xu LP, Jin S, Wang SQ, et al. Upfront haploidentical transplant for acquired severe aplastic anemia: Registry-based comparison with matched related transplant[J]. J Hematol Oncol, 2017, 10(1): 25. doi: 10.1186/s13045-017-0398-y
[20] Ma X, Zuo Y, Xu Z, et al. Comparable clinical outcomes of haploidentical hematopoietic stem cell transplantation in patients with hepatitis-associated aplastic anemia and non-hepatitis-associated aplastic anemia[J]. Ann Hematol, 2022, 101(8): 1815-1823. doi: 10.1007/s00277-022-04885-w
[21] Vaht K, Goransson M, Carlson K, et al. Low response rate to ATG-based immunosuppressive therapy in very severe aplastic anaemia-A Swedish nationwide cohort study[J]. Eur J Haematol, 2018, 100(6): 613-620. doi: 10.1111/ejh.13057
[22] Liu J, Lu XY, Cheng L, et al. Clinical outcomes of immunosuppres sive therapy for severe aplastic anemia patients with absolute neutro phil count of zero[J]. Hematology, 2019, 24(1): 492-497. doi: 10.1080/16078454.2019.1631424
-




 下载:
下载: