Therapeutic drug monitoring of venetoclax in Chinese acute myeloid leukemia patients using venetoclax combined with azacitidine
-
摘要: 目的 探讨维奈克拉(venetoclax,VEN)联合阿扎胞苷(azacitidine,AZA)治疗中国急性髓系白血病(acute myeloid leukemia,AML)患者中VEN血药浓度监测以及与疗效的关系。方法 回顾性分析2021年4月至2023年2月于北京大学血液病研究所接受VEN联合AZA治疗和持续性VEN血药浓度监测的38例初治不适合强化疗(unfit)及难治/复发(R/R)AML患者。分析真实世界中国人群第1个疗程VEN联合AZA诱导治疗AML期间持续性监测d4、d7、d14、d21、d28 VEN谷浓度及与疗效的关系,以及与可能影响VEN血药浓度的基线患者情况和基线临床特征的相关性。结果 入组38例AML患者,初治unfit AML 21例(55.3%),R/R AML 17例(44.7%),中位年龄51.5(16~70)岁,男19例(50.0%),中位随访时间18.9(5.5~28.1)个月。所有患者均因联合应用伏立康唑而调整VEN剂量为100 mg Qd。根据2022 ELN标准进行危险分层,初治unfit组高危15例(71.4%),R/R组高危9例(52.9%)。初治unfit组VEN联合AZA中位治疗疗程为1(1~5)个,R/R组VEN联合AZA中位治疗疗程为1(1~2)个。经1个疗程VEN联合AZA治疗后,初治unfit AML患者14例(66.7%)达完全缓解/血细胞计数未完全恢复的完全缓解(CR/CRi),4例(19.0%)达部分缓解(PR),总体反应率达85.7%(18/21)。R/R AML患者6例(35.3%)达CR/CRi,4例(23.5%)达PR,总体反应率达58.8%(10/17)。初治unfit组诱导期间VEN稳态谷浓度Cmin在d4、d7、d14、d21、d28分别为1 330(299~6 570) ng/mL、2 158(264~5 050) ng/mL、2 404(533~5 760) ng/mL、2 752(282~4 850) ng/mL、2 251(560~4 410) ng/mL。R/R组再诱导期间VEN稳态谷浓度Cmin分别为1 203(692~3 100) ng/mL、2 245(799~5 580) ng/mL、1 950(890~4 920) ng/mL、1 856(473~3 210) ng/mL、1 650(934~2 584) ng/mL。结果显示VEN谷浓度Cmin在初治unfit组(P=0.23)及R/R组(P=0.24)有反应与未缓解组间均差异无统计学意义。VEN谷浓度与患者基线特征(性别、年龄、身高、体重、体重指数、体表面积)及基线临床特征(白蛋白、丙氨酸氨基转移酶、天冬氨酸氨基转移酶、血尿素氮、肌酐、乳酸脱氢酶、中性粒细胞绝对计数、白细胞计数、血红蛋白水平及血小板计数)无明显相关性。结论 VEN联合AZA在初治unfit及R/R AML患者中均获得较高的治疗反应率,VEN稳态谷浓度和临床疗效可能无显著相关。Abstract: Objective To investigate the monitoring of drug plasma concentration of venetoclax(VEN) and its relationship with therapeutic efficacy in Chinese acute myeloid leukemia(AML) patients receiving VEN combined with azacitidine(AZA).Methods A total of 38 AML patients receiving VEN+AZA and undergoing continuous VEN drug plasma concentration monitoring between April 2021 and February 2023 at Peking University Institute of Hematology were retrospectively analyzed. We analyzed continuously monitoring VEN trough concentration on d4, d7, d14, d21 and d28 during the first course of VEN+AZA induction therapy for AML in the real-world Chinese population and its relationship with therapeutic effect, as well as the correlation with the baseline patient conditions and clinical characteristics that may influence VEN drug plasma concentration.Results A total of 38 AML patients were enrolled, including 21(55.3%) unfit de novo AML cases and 17(44.7%) R/R AML cases. The median age was 51.5 years(range 16-70 years), and 19 patients(50.0%) were male. The median follow-up time was 18.9 months(range 5.5-28.1 months). All patients received VEN at an adjusted dose of 100 mg Qd due to the combination of voriconazole. According to the 2022 ELN risk group classification, 15 patients(71.4%) in the unfit de novo AML group were high-risk group, and 9 patients(52.9%) in R/R AML group were high-risk group. The median treatment duration was 1 course(range 1-5 courses) for the unfit de novo AML group and 1 course(range 1-2 courses) for R/R AML group. After one cycle of VEN+AZA, for the unfit de novo AML group, 14 patients(66.7%) achieved complete remission(CR)/CR with incomplete hematological recovery(CRi), and 4 patients(19.0%) achieved partial remission(PR), with an overall response rate(ORR) of 85.7%(18/21). For R/R AML group, six patients(35.3%) achieved CR/CRi, 4 patients(23.5%) achieved PR, and the ORR was 58.8%(10/17). The VEN steady-state Cmin of unfit patients during induction therapy were 1 330(299-6 570) ng/mL, 2 158(264-5 050) ng/mL, 2 404(533-5 760) ng/mL, 2 752(282-4 850) ng/mL, 2 251(560-4 410) ng/mL on d4, d7, d14, d21 and d28, respectively. The VEN steady-state Cmin of R/R patients during re-induction therapy were 1 203(692-3 100) ng/mL, 2 245(799-5 580) ng/mL, 1 950(890-4 920) ng/mL, 1 856(473-3 210) ng/mL, 1 650(934-2 584) ng/mL, respectively. The results showed there was no significant difference in the Cmin of VEN between patients achieving ORR and no response in unfit(P=0.23) and R/R patients(P=0.24). The Cmin of VEN were not significantly correlated with baseline patient characteristics(gender, age, height, weight, body mass index, and body surface area) or baseline clinical features(albumin, alanine aminotransferase, aspartate aminotransferase, blood urea nitrogen, creatinine, lactate dehydrogenase, absolute neutrophil count, white blood cell count, hemoglobin level, and platelet count).Conclusion VEN+AZA achieved high therapeutic response rates in both unfit and R/R AML patients, and there may be no significant correlation between the VEN steady-state Cmin and clinical efficacy.
-
急性髓系白血病(acute myeloid leukemia,AML)是成人最常见的急性白血病,5年生存率约为27%[1-2]。“3+7”诱导化疗是AML的标准治疗[3],但仍有超过45%的AML患者不适合强化疗[4]。维奈克拉(venetoclax,VEN)是高选择性小分子靶向BCL-2抑制剂[5]。VEN联合去甲基化药物或低剂量阿糖胞苷已成为不适合强化疗(unfit)及老年AML的一线标准治疗方案[6-7],也是难治/复发(R/R)AML的有效选择之一[8-10]。然而,真实世界中国AML患者的VEN血药浓度与疗效是否有关仍不明确。本研究分析了VEN联合阿扎胞苷(azacitidine,AZA)治疗AML的疗效和安全性,探索了真实世界中国人群中多个时间点持续监测的VEN稳态谷浓度Cmin与疗效的关系以及影响VEN浓度的相关因素。
1. 资料与方法
1.1 资料
纳入2021年4月1日至2023年2月28日北京大学人民医院血液科接受至少1个疗程VEN联合AZA治疗且接受连续VEN血药浓度监测的AML患者共38例,其中初治unfit组21例,R/R组17例。AML诊断根据2022版WHO髓样肿瘤和急性白血病的分型诊断标准。依据欧洲白血病网络(ELN)2022标准进行危险分层。所有患者经骨髓细胞形态学、免疫分型、细胞遗传学、分子生物学等检查确诊[11-12]。本研究根据《赫尔辛基宣言》进行,并经北京大学人民医院伦理委员会批准。
1.2 治疗药物
患者在第1个疗程接受VEN剂量爬坡,VEN 100 mg第1天,200 mg第2天,400 mg第3~28天,口服。从第2个疗程开始,直接予每日400 mg口服。根据骨髓抑制情况和药物相互作用调整用药量,粒细胞缺乏期均采用伏立康唑预防或治疗真菌感染,VEN维持剂量降低为100 mg/d。治疗方案中联合应用去甲基化药物AZA(75 mg/m2),在所有疗程的第1~7天通过皮下注射给药,每日1次。如出现难以控制的活动性感染、脏器损伤,治疗中止。
1.3 定义
1.3.1 初治unfit的标准
至少满足以下条件之一:①≥75岁;②美国东部肿瘤协作组(ECOG)体能状态评分为2~3分;③严重的心、肺、肝或肾脏疾病;④对抗感染治疗无效的活动性感染;⑤存在认知障碍;⑥存在医生判断不适合接受强化化疗的合并症[13-14]。
1.3.2 难治
≥2个标准方案化疗后未达完全缓解(CR)状态。
1.3.3 复发
达CR的患者外周血中又出现白血病细胞或骨髓中原始细胞≥5%或髓外白血病细胞浸润。
1.3.4 疗效评估
CR:骨髓原始细胞 < 5%,原始细胞内不含有Auer小体;外周血无原始细胞;无髓外白血病;外周血绝对中性粒细胞计数(ANC)>1.0×109/L,且血小板计数(PLT)>100×109/L。CR伴血液学不完全恢复(CRi):外周血ANC < 1.0×109/L或PLT < 100×109/L,其他满足CR的标准。部分缓解(PR):化疗后骨髓原始细胞比例减少至少50%,且下降到5%~25%,外周血细胞计数正常。总体反应率(ORR)定义为患者在治疗后达CR、CRi和PR的比例。未缓解(NR):诱导治疗后未获得CR或PR。总生存期(OS)指从开始应用VEN至随访截止或患者因任何原因死亡的时间。无病生存期(DFS)指患者达CR至复发、死亡或随访截止的时间。
1.3.5 不良反应
所有不良反应按照NCI常见不良反应事件术语标准5.0版本进行报告和分级。
1.4 VEN治疗性血药浓度监测
1.4.1 患者基线特征和基线临床特征
患者基线特征包括性别、年龄、身高、体重、体重指数(BMI)、体表面积(BSA);基线临床特征包括白蛋白(ALB)、丙氨酸氨基转移酶(ALT)、天冬氨酸氨基转移酶(AST)、血尿素氮(BUN)、肌酐(CREA)、乳酸脱氢酶(LDH)、ANC、白细胞计数(WBC)、血红蛋白(Hb)及PLT。
1.4.2 VEN血药浓度监测
在诱导治疗期间VEN血药浓度达到稳态(即d3后)d4、d7、d14、d21和d28,监测给药前0.5 h外周血血药浓度,记为Cmin。采用HPLC-MS/MS法进行血药浓度测定,所用仪器和试剂为岛津Nexera Xp型高效液相色谱仪(日本岛津公司),API3200 MD三重四级杆质谱仪(美国AB SCIEX公司),质谱仪采用电喷雾(ESI)离子源;标准品VEN,内标VEN-d8。
1.5 随访
通过电话或查阅门诊和住院病历的方式进行随访。随访截至2023年7月31日。
1.6 统计学处理
采用R4.2.2软件进行统计学分析。分类变量以例(%)表示,采用χ2检验和Fisher确切概率法进行分析;符合正态分布的连续变量以 X±S表示,组间比较采用独立样本t检验;不符合正态分布的连续变量以中位数(范围)表示,组间比较采用Mann-Whitney U检验和Kruskal-Wallis检验。检验水准α=0.05,以P < 0.05为差异有统计学意义。使用Kaplan-Meier曲线分析OS和DFS,并使用对数秩检验根据感兴趣的因素进行分层。为了评估临床和生物学特征对VEN血药浓度的影响,采用Pearson相关性或Spearman相关性分析探索影响VEN谷浓度的因素。
2. 结果
2.1 患者特征
入组38例患者,初治unfit组21例(55.3%),R/R组17例(44.7%)。所有患者均因联合应用伏立康唑而调整VEN剂量为100 mg Qd。入组患者中位年龄51.5(16~70)岁,男19例(50.0%)。根据2022 ELN标准进行危险分层,初治unfit组高危15例(71.4%),R/R组高危9例(52.9%)。初治unfit组中位治疗疗程为1(1~5)个,R/R组中位治疗疗程为1(1~2)个。截至2023年7月31日,初治组及R/R组中位随访时间分别为18.9(5.7~28.1)个月和18.0(5.5~27.1)个月。初治unfit组和R/R组患者的基线特征见表 1。
表 1 38例接受VEN联合AZA治疗AML患者的基线特征指标 初治unfit组(21例) R/R组(17例) 性别/例(%) 女 10(47.6) 9(52.9) 男 11(52.4) 8(47.1) 中位年龄(范围)/岁 58(16~70) 34(16~59) ECOG评分/例(%) 0 0 1(5.9) 1 4(19.0) 5(29.4) 2 10(47.6) 6(35.3) 3 7(33.3) 5(29.4) FAB分型/例(%) M1 1(4.8) 0 M2 14(66.7) 8(47.1) M4 5(23.8) 6(35.3) M5 1(4.8) 2(11.8) M6 0 1(5.9) 2022 ELN危险分层/例(%) 低危 0 3(17.6) 中危 6(28.6) 5(29.4) 高危 15(71.4) 9(52.9) 特殊基因标志/例(%) NPM1 0 1(5.9) IDH1/IDH2 3(14.3) 0 TP53 3(14.3) 0 FLT3-ITD 0 3(17.6) RUNX1-RUNX1T1 1(4.8) 3(17.6) MLL 1(4.8) 1(5.9) CEBPA框突变 1(4.8) 4(23.5) 序贯移植/例(%) 8(38.1) 8(47.1) 身高/cm 165(146~183) 165(152~185) 体重/kg 65(42.5~105) 59(45~80) BMI/(kg/m2) 23.6 (17.9~33.5) 21.7 (16.1~27.9) BSA/m2 1.69 (1.31~2.27) 1.60 (1.35~1.95) ALB/(g/L) 36.2 (30.2~42.7) 37.2 (31.4~45.4) ALT/(U/L) 15.9 (8.9~61.0) 19.2 (5.2~327.1) AST/(U/L) 20.3 (8.9~40.6) 17.2 (9.5~247.8) BUN/(mmol/L) 5.29 (2.67~17.89) 4.60 (1.22~7.65) CREA/(μmol/L) 61.7 (35.8~182.1) 57.5 (38.8~100.4) LDH/(U/L) 247 (94~769) 194 (117~685) ANC/(×109/L) 0.13 (0.01~9.79) 0.58 (0.04~31.00) WBC/(×109/L) 3.42 (0.41~85.40) 15.82 (1.82~222.05) Hb/(g/L) 70(49~131) 81(43~136) PLT/(×109/L) 62(6~408) 38(4~273) 2.2 疗效及生存分析
21例初治unfit AML患者中17例诊断时合并活动性感染,4例合并肾功能不全。所有患者均完成28 d诱导治疗。经1个疗程VEN联合AZA治疗后,14例(66.7%)达CR/CRi,4例(19.0%)达PR,ORR达85.7%(18/21)。中危组(6例)接受1个疗程VEN联合AZA后,83.3%达CR/CRi,高危组(15例)接受1个疗程VEN联合AZA后,60.0%达CR/CRi。5例患者接受2个疗程VEN联合AZA,6例接受3个疗程VEN联合AZA,1例接受5个疗程VEN联合AZA。16例CR/CRi患者中13例(81.3%)经1~2个疗程治疗后微小残留病灶(MRD)转阴。8例患者经VEN联合AZA诱导达CR/CRi后,unfit因素(不易控制的活动性感染)消除,后衔接标准化疗巩固治疗,经1~2个疗程巩固化疗后MRD均转阴。8例(38.1%)患者后续接受异基因造血干细胞移植(allo-HSCT),全部为诱导治疗后达CR/CRi的患者。初治unfit组中位随访时间为18.9(5.7~28.1)个月,2年DFS率为73.4%,2年OS率为78.6%。
17例R/R组AML患者使用VEN联合AZA治疗1个疗程后,6例(35.3%)达CR/CRi,4例(23.5%)达PR,ORR达58.8%(10/17)。7例患者接受了第2个疗程VEN联合AZA。6例CR/CRi患者中4例(66.7%)经1~2个疗程治疗后MRD转阴。8例(47.1%)患者后续接受allo-HSCT,其中5例患者诱导治疗达CR/CRi后桥接allo-HSCT,3例为诱导治疗NR后接受挽救性allo-HSCT。R/R组中位随访时间为18.0(5.5~27.1)个月,2年DFS率为66.7%,2年OS率为55.0%。
2.3 安全性
血液学不良反应发生率为97.4%(37/38),其中中性粒细胞减少伴发热的发生率为63.2%(24/38)。最常见的非血液学不良反应为胃肠道反应,主要包括恶心呕吐(18.4%)及腹泻(5.3%)。3例(7.9%)患者出现肝功能损伤,1例(2.6%)患者出现肿瘤溶解综合征。
2.4 VEN谷浓度与临床疗效及患者特征相关性结果
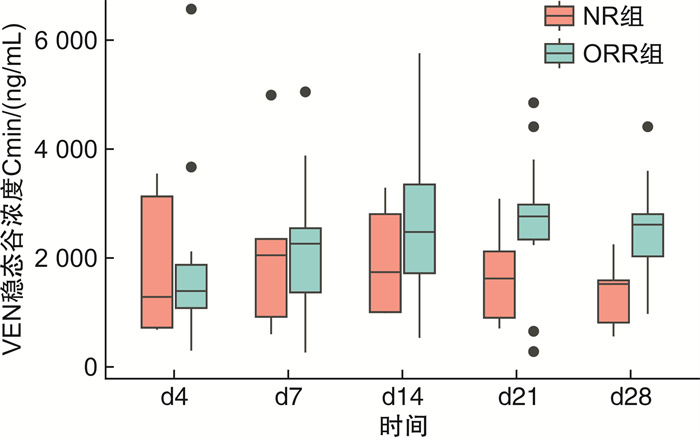
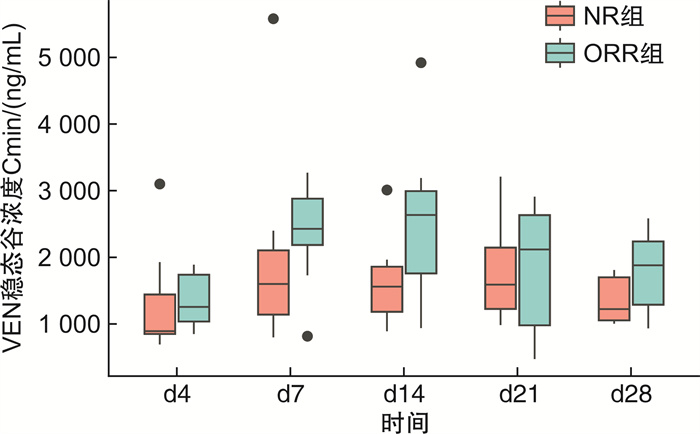
38例患者在诱导d4、d7、d14、d21和d28的VEN稳态谷浓度Cmin范围在1 593~2 320 ng/mL。初治unfit组诱导期间VEN稳态谷浓度Cmin在d4、d7、d14、d21和d28分别为1 330(299~6 570) ng/mL、2 158(264~5 050) ng/mL、2 404(533~5 760) ng/mL、2 752(282~4 850) ng/mL、2 251(560~4 410) ng/mL。初治unfit组VEN稳态谷浓度Cmin在d4、d7、d14、d21和d28差异无统计学意义(P=0.13)。初治unfit组在诱导d4、d7、d14、d21和d28的VEN稳态谷浓度Cmin平均值范围在1 794~2 467 ng/mL。R/R组再诱导期间VEN稳态谷浓度Cmin在d4、d7、d14、d21和d28分别为1 203(692~3 100) ng/mL、2 245(799~5 580) ng/mL、1 950(890~4 920) ng/mL、1 856(473~3 210) ng/mL、1 650(934~2 584) ng/mL。R/R组VEN稳态谷浓度Cmin在d4、d7、d14、d21和d28差异有统计学意义(P=0.03)。R/R组在诱导d4、d7、d14、d21和d28的VEN稳态谷浓度Cmin平均值范围在1 345~2 256 ng/mL。38例AML患者接受VEN联合AZA诱导治疗期间的VEN谷浓度见表 2。
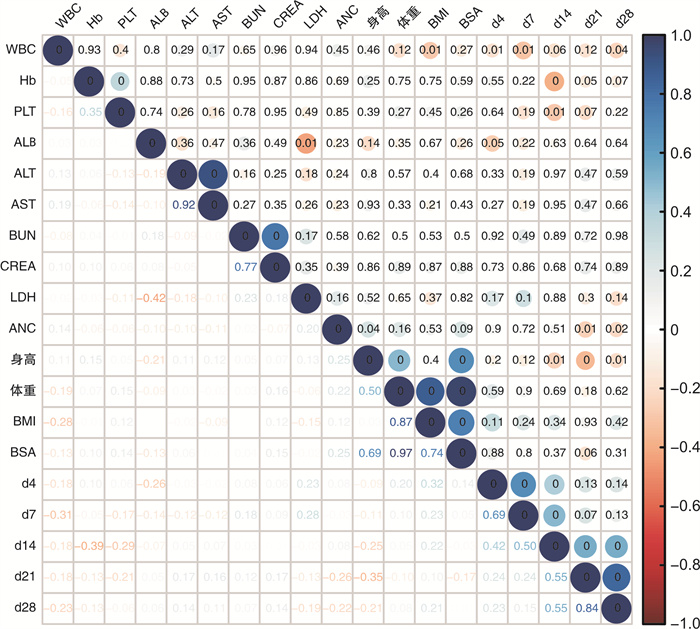
表 2 38例患者诱导期间的VEN谷浓度Cminng/mL 时间 初治unfit组(21例) R/R组(17例) 中位数 范围 中位数 范围 d4 1 330 299~6 570 1 203 692~3 100 d7 2 158 264~5 050 2 245 799~5 580 d14 2 404 533~5 760 1 950 890~4 920 d21 2 752 282~4 850 1 856 473~3 210 d28 2 251 560~4 410 1 650 934~2 584 分别比较了初治unfit组及R/R组中达到ORR组与NR组间VEN谷浓度的差异,结果显示VEN谷浓度Cmin在初治unfit组及R/R组达到ORR组与NR组间均差异无统计学意义。初治unfit组患者在接受VEN联合AZA诱导期间,d4、d7、d14、d21、d28的VEN谷浓度在达到ORR组与NR组间差异无统计学意义(P=0.23)(图 1)。R/R组患者在接受VEN联合AZA再诱导期间,d4、d7、d14、d21、d28的VEN谷浓度在达到ORR组与NR组间差异亦无统计学意义(P=0.24)(图 2)。热图显示了VEN血药浓度与基线患者临床特征间的相关性分析(图 3),结果显示患者身高、体重、BMI、BSA、基线ALB、ALT、AST、BUN、CREA、LDH、ANC、WBC、Hb及PLT与VEN血药浓度无明显相关性。
3. 讨论
本研究回顾性分析了VEN联合AZA治疗AML患者的临床疗效及安全性,更重要的是,探索性分析了在真实世界中国人群使用VEN诱导治疗期间d4、d7、d14、d21和d28的持续血药浓度水平及其与疗效间的关系,以及可能影响VEN血药浓度的患者临床因素。既往研究仅关注于中国AML患者单个时间点的VEN峰浓度Cmax和谷浓度Cmin的监测[15-16]。由于VEN联合AZA是28 d长疗程的诱导化疗方案,血药浓度和个体变异性大,目前尚不明确单个时间点的血药浓度能否反映长疗程中VEN血药浓度的真实变化,多个时间点连续监测VEN血药浓度可能更有利于观察浓度和疗效间的相关性。
本研究我们观察到在真实世界中国AML患者使用VEN联合AZA诱导期间的VEN谷浓度Cmin平均值范围在1 593~2 320 ng/mL;类似地,Yang等[16]采用LC-MS/MS法测定中国AML患者VEN药代动力学参数的结果显示,VEN的Cmin为(1 018.0±729.4)ng/mL。Kobayashi等[17]报道了日本AML患者的VEN的Cmin范围在0.39~2.49 μg/mL。因此,VEN在亚裔人群相比既往报道在非亚裔人群观察到的Cmin范围在0.69~0.99 μg/mL更高[18]。本研究观察到Cmin有异常值现象,最高值为1例初治unfit AML患者,第14天测定Cmin高达5 760 ng/mL,该患者在接受2个疗程VEN联合AZA治疗后,骨髓评估达CR,在诱导化疗过程中发生4级血液学毒性,但未出现感染。这提示单一时间点谷浓度测定能否反映VEN的28 d长疗程中血药浓度的真实变化仍存在争议。由于VEN联合AZA是一种28 d长疗程的诱导化疗方案,血药浓度波动大,个体变异性大,因此尚不清楚单个时间点的浓度能否反映28 d长疗程中药物血药浓度的真实变化,及其是否与疗效有关。因此,在多个时间点连续监测VEN血药浓度可能更好地反映真实世界中VEN的血药浓度变化,更有利于探索血药浓度与疗效间的关系。
Brackman等[19]研究认为虽然亚裔AML患者的VEN生物利用度比非亚裔患者高67%,但亚裔患者总体的个体暴露范围与非亚裔AML患者相当。同时考虑到在接受VEN治疗的AML患者中未发现有显著的暴露-疗效关系,提示VEN在达到目标剂量时其临床疗效已最优化,支持在临床实践中采用VEN固定剂量的方式给药。本研究中所有患者均因联合应用伏立康唑而按照固定剂量100 mg Qd口服VEN。本研究我们观察到在初治unfit AML患者接受1个疗程VEN联合AZA后CR/CRi率达67.7%,R/R组1个疗程后CR/CRi率达35.3%。这表明VEN联合AZA的方案在初治unfit及R/R AML患者中显示出良好的疗效及耐受性,与既往的临床研究及其他真实世界研究数据一致[20-24]。此外,在初治unfit组及R/R组均未发现VEN血药浓度与临床疗效间存在显著相关性。因此,本研究提示在中国人群中基于VEN血药浓度监测来调整VEN剂量的证据可能不足。
本研究报道了中国AML患者接受VEN联合AZA诱导治疗期间临床特征,包括患者身高、体重、BMI、BSA、治疗时基线ALB、ALT、AST、BUN、CREA、LDH、ANC、WBC、Hb及PLT与VEN在诱导治疗期间连续性血药浓度监测的相关性分析。热图结果显示,患者VEN血药浓度与上述可能影响药物在体内代谢变异度的临床特征因素未有显著相关性。Yang等[16]研究发现在62例中国AML患者中,VEN的Cmin与AST和ALT水平呈正相关。然而,Brackman等[19]研究报道的基于771例接受VEN联合去甲基化药物或低剂量阿糖胞苷的AML患者的群体药代动力学模型显示,VEN血药浓度与其在体内的暴露不随年龄、体重、性别、轻度至中度肝损伤或轻度至重度肾损伤而发生显著变化。需要进一步大样本、多中心的研究证实这个结论。
在药代动力学领域,通常使用曲线下面积表示药物在体内的真实暴露水平。然而,在目前的临床实践中测定VEN的曲线下面积的可行性有限,因为每例患者需要在服药后的1 d内多次采样,这在真实世界中很难实现。因此,本研究选择测定d4、d7、d14、d21和d28的瞬时谷浓度Cmin来表示VEN在体内的暴露浓度。考虑达峰时间可能受个体变异度的影响,未测定VEN的峰浓度Cmax,这是本文的局限性之一;另外,鉴于本文纳入样本量少、随访时间短,因此研究结果仍有待更大规模样本量的临床研究进行进一步证实。
总之,VEN联合AZA在初治unfit及R/R AML患者中均有效,VEN稳态谷浓度和临床疗效可能无显著相关。期待大规模、多中心的研究进一步展示和证实中国人群VEN稳态谷浓度及其与疗效的关系以及影响VEN血药浓度的临床相关因素。
利益冲突 所有作者均声明不存在利益冲突
-
表 1 38例接受VEN联合AZA治疗AML患者的基线特征
指标 初治unfit组(21例) R/R组(17例) 性别/例(%) 女 10(47.6) 9(52.9) 男 11(52.4) 8(47.1) 中位年龄(范围)/岁 58(16~70) 34(16~59) ECOG评分/例(%) 0 0 1(5.9) 1 4(19.0) 5(29.4) 2 10(47.6) 6(35.3) 3 7(33.3) 5(29.4) FAB分型/例(%) M1 1(4.8) 0 M2 14(66.7) 8(47.1) M4 5(23.8) 6(35.3) M5 1(4.8) 2(11.8) M6 0 1(5.9) 2022 ELN危险分层/例(%) 低危 0 3(17.6) 中危 6(28.6) 5(29.4) 高危 15(71.4) 9(52.9) 特殊基因标志/例(%) NPM1 0 1(5.9) IDH1/IDH2 3(14.3) 0 TP53 3(14.3) 0 FLT3-ITD 0 3(17.6) RUNX1-RUNX1T1 1(4.8) 3(17.6) MLL 1(4.8) 1(5.9) CEBPA框突变 1(4.8) 4(23.5) 序贯移植/例(%) 8(38.1) 8(47.1) 身高/cm 165(146~183) 165(152~185) 体重/kg 65(42.5~105) 59(45~80) BMI/(kg/m2) 23.6 (17.9~33.5) 21.7 (16.1~27.9) BSA/m2 1.69 (1.31~2.27) 1.60 (1.35~1.95) ALB/(g/L) 36.2 (30.2~42.7) 37.2 (31.4~45.4) ALT/(U/L) 15.9 (8.9~61.0) 19.2 (5.2~327.1) AST/(U/L) 20.3 (8.9~40.6) 17.2 (9.5~247.8) BUN/(mmol/L) 5.29 (2.67~17.89) 4.60 (1.22~7.65) CREA/(μmol/L) 61.7 (35.8~182.1) 57.5 (38.8~100.4) LDH/(U/L) 247 (94~769) 194 (117~685) ANC/(×109/L) 0.13 (0.01~9.79) 0.58 (0.04~31.00) WBC/(×109/L) 3.42 (0.41~85.40) 15.82 (1.82~222.05) Hb/(g/L) 70(49~131) 81(43~136) PLT/(×109/L) 62(6~408) 38(4~273) 表 2 38例患者诱导期间的VEN谷浓度Cmin
ng/mL 时间 初治unfit组(21例) R/R组(17例) 中位数 范围 中位数 范围 d4 1 330 299~6 570 1 203 692~3 100 d7 2 158 264~5 050 2 245 799~5 580 d14 2 404 533~5 760 1 950 890~4 920 d21 2 752 282~4 850 1 856 473~3 210 d28 2 251 560~4 410 1 650 934~2 584 -
[1] Pulte D, Jansen L, Brenner H. Changes in long term survival after diagnosis with common hematologic malignancies in the early 21st century[J]. Blood Cancer J, 2020, 10(5): 56. doi: 10.1038/s41408-020-0323-4
[2] Song X, Peng Y, Wang X, et al. Incidence, Survival, and Risk Factors for Adults with Acute Myeloid Leukemia Not Otherwise Specified and Acute Myeloid Leukemia with Recurrent Genetic Abnormalities: Analysis of the Surveillance, Epidemiology, and End Results(SEER)Database, 2001-2013[J]. Acta Haematol, 2018, 139(2): 115-127. doi: 10.1159/000486228
[3] Short NJ, Rytting ME, Cortes JE. Acute myeloid leukaemia[J]. Lancet, 2018, 392(10147): 593-606. doi: 10.1016/S0140-6736(18)31041-9
[4] Mamolo CM, Cappelleri JC, Hoang CJ, et al. A real-world, cross-sectional, community survey of symptoms and health-related quality of life of adults with acute myeloid leukemia[J]. Future Oncol, 2019, 15(16): 1895-1909. doi: 10.2217/fon-2018-0842
[5] Lachowiez C, Dinardo CD, Konopleva M. Venetoclax in acute myeloid leukemia-current and future directions[J]. Leuk Lymphoma, 2020, 61(6): 1313-1322. doi: 10.1080/10428194.2020.1719098
[6] 中华医学会血液学分会白血病淋巴瘤学组. 中国成人急性髓系白血病(非急性早幼粒细胞白血病)诊疗指南(2021年版)[J]. 中华血液学杂志, 2021, 42(8): 617-623.
[7] Pollyea DA, Bixby D, Perl A, et al. NCCN Guidelines Insights: Acute Myeloid Leukemia, Version 2.2021[J]. J Natl Compr Canc Netw, 2021, 19(1): 16-27. doi: 10.6004/jnccn.2021.0002
[8] Bewersdorf JP, Giri S, Wang R, et al. Venetoclax as monotherapy and in combination with hypomethylating agents or low dose cytarabine in relapsed and treatment refractory acute myeloid leukemia: a systematic review and meta-analysis[J]. Haematologica, 2020, 105(11): 2659-2663. doi: 10.3324/haematol.2019.242826
[9] Feld J, Tremblay D, Dougherty M, et al. Safety and Efficacy: Clinical Experience of Venetoclax in Combination With Hypomethylating Agents in Both Newly Diagnosed and Relapsed/Refractory Advanced Myeloid Malignancies[J]. Hemasphere, 2021, 5(4): e549. doi: 10.1097/HS9.0000000000000549
[10] Dinardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia[J]. Blood, 2019, 133(1): 7-17. doi: 10.1182/blood-2018-08-868752
[11] Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN[J]. Blood, 2022, 140(12): 1345-1377. doi: 10.1182/blood.2022016867
[12] Arber DA, Orazi A, Hasserjian RP, et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data[J]. Blood, 2022, 140(11): 1200-1228. doi: 10.1182/blood.2022015850
[13] Ferrara F, Barosi G, Venditti A, et al. Consensus-based definition of unfitness to intensive and non-intensive chemotherapy in acute myeloid leukemia: a project of SIE, SIES and GITMO group on a new tool for therapy decision making[J]. Leukemia, 2013, 27(5): 997-999. doi: 10.1038/leu.2012.303
[14] Senapati J, Dhawan R, Aggarwal M, et al. Venetoclax and azacitidine(VenAZA)combination therapy in young unfit patients with AML: a perspective from a developing country[J]. Leuk Lymphoma, 2021, 62(6): 1514-1517. doi: 10.1080/10428194.2020.1867724
[15] Gao P, Zhang W, Fang X, et al. Simultaneous quantification of venetoclax and voriconazole in human plasma by UHPLC-MS/MS and its application in acute myeloid leukemia patients[J]. J Pharm Biomed Anal, 2023, 227: 115279. doi: 10.1016/j.jpba.2023.115279
[16] Yang X, Mei C, He X, et al. Quantification of Venetoclax for Therapeutic Drug Monitoring in Chinese Acute Myeloid Leukemia Patients by a Validated UPLC-MS/MS Method[J]. Molecules, 2022, 27(5): 1607. doi: 10.3390/molecules27051607
[17] Kobayashi M, Yasu T, Suzaki K, et al. Utility of therapeutic drug monitoring of venetoclax in acute myeloid leukemia[J]. Med Oncol, 2022, 39(12): 259. doi: 10.1007/s12032-022-01865-y
[18] Salem AH, Dunbar M, Agarwal SK. Pharmacokinetics of venetoclax in patients with 17p deletion chronic lymphocytic leukemia[J]. Anticancer Drugs, 2017, 28(8): 911-914. doi: 10.1097/CAD.0000000000000522
[19] Brackman D, Eckert D, Menon R, et al. Venetoclax exposure-efficacy and exposure-safety relationships in patients with treatment-naïve acute myeloid leukemia who are ineligible for intensive chemotherapy[J]. Hematol Oncol, 2022, 40(2): 269-279. doi: 10.1002/hon.2964
[20] 龚敏, 吴迪, 李秋柏, 等. 维奈克拉治疗急性髓系白血病的疗效与安全性的单中心真实世界研究[J]. 临床血液学杂志, 2022, 35(5): 359-363. doi: 10.13201/j.issn.1004-2806.2022.05.012
[21] Winters AC, Gutman JA, Purev E, et al. Real-world experience of venetoclax with azacitidine for untreated patients with acute myeloid leukemia[J]. Blood Adv, 2019, 3(20): 2911-2919. doi: 10.1182/bloodadvances.2019000243
[22] 盘婉盈, 张映璇, 涂三芳, 等. Venetoclax联合去甲基化药物治疗复发/难治性急性髓系白血病的疗效及预后分析[J]. 临床血液学杂志, 2021, 34(9): 650-654. doi: 10.13201/j.issn.1004-2806.2021.09.010
[23] 李可芯, 赵薇薇, 刘瑶, 等. 维奈克拉血药浓度监测在治疗急性髓系白血病中的价值及联合阿扎胞苷治疗急性髓系白血病的疗效与安全性分析[J]. 临床血液学杂志, 2022, 35(11): 812-816. doi: 10.13201/j.issn.1004-2806.2022.11.012
[24] Dinardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia[J]. N Engl J Med, 2020, 383(7): 617-629. doi: 10.1056/NEJMoa2012971
-




 下载:
下载: