Analysis of the efficacy and safety of a long course of venetoclax in the treatment of patients with acute myeloid leukemia
-
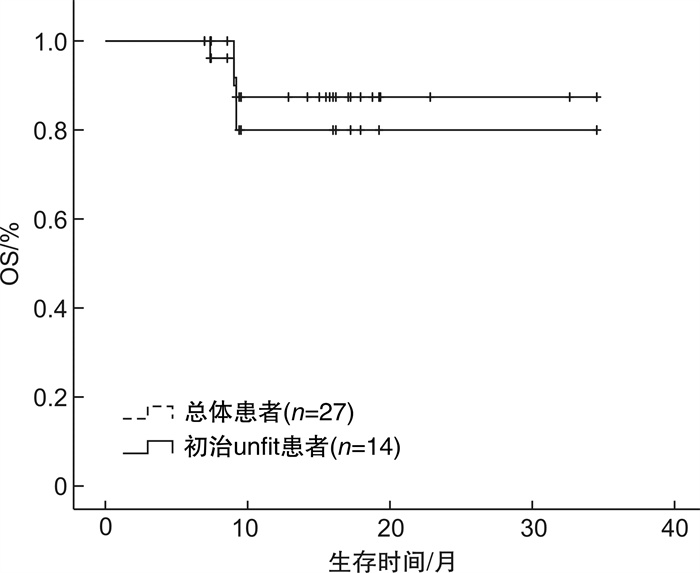
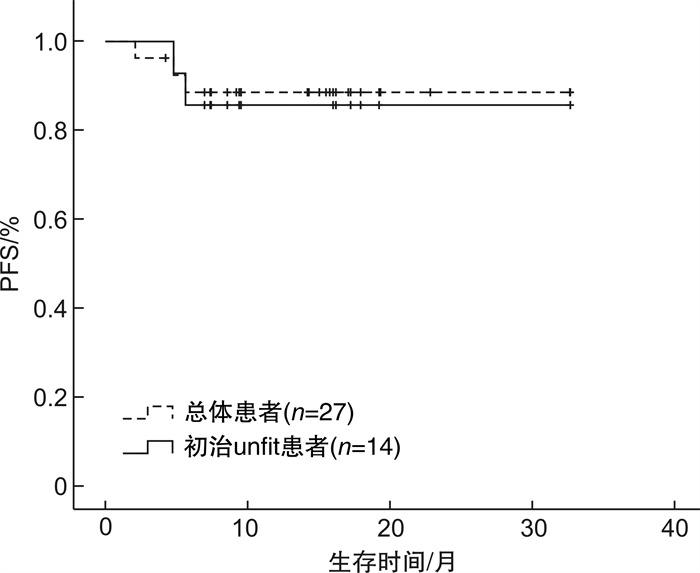
摘要: 目的 探讨维奈克拉联合方案长疗程治疗急性髓系白血病(acute myeloid leukemia,AML)患者的疗效和安全性,以指导更加个体化的临床应用。方法 回顾性分析2021年1月至2023年7月于我院接受维奈克拉联合方案长疗程(≥6个周期)治疗的AML患者的病历资料,统计患者的复合完全缓解率、无进展生存期、总生存期以及不良反应发生情况。结果 共纳入27例患者,中位随访时间为15(9.2~17.9)个月,维奈克拉联合治疗的中位周期数为8(6~28)个周期。在结束2个周期治疗后24例患者可评估疗效,复合完全缓解率为91.67%,部分缓解率为4.17%。所有患者首次获得复合完全缓解率的中位周期为1(1~2)个周期,随访期内55.56%的患者获得持续缓解。17例患者有微小残留病结果,微小残留病转阴率为58.82%。33.33%的患者微小残留病持续阴性,获得了持久而深度的缓解。27例患者中6例(22.22%)出现疾病进展。中位无进展生存期及总生存期均未达到,1年总生存率为84.21%。安全性分析以血液学不良反应最常见,其次为消化道症状、电解质紊乱、肺部感染、肝功异常等。结论 维奈克拉长疗程治疗AML具有良好的有效性及安全性,部分患者可达深度而持久的缓解,维奈克拉可作为AML患者巩固及维持治疗的选择。Abstract: Objective To investigate the efficacy and safety of a long course of the venetoclax combination regimen in the treatment of patients with acute myeloid leukemia(AML), in order to guide a more individualized clinical application.Methods The medical records of AML patients who received a long course of treatment(≥6 cycles) with the combination regimen of venetoclax from January 2021 to July 2023 were retrospectively analyzed for the composite complete remission(cCR), progression-free survival(PFS), overall survival(OS), and the occurrence of adverse events.Results A total of 27 patients were included with a median follow-up of 15(9.2-17.9) months and a median number of cycles of VEN combination therapy of 8(6-28) cycles. After 2 cycles of treatment, 24 patients were evaluated for efficacy, with a cCR of 91.67% and a partial remission of 4.17%. The median number of cycles to first cCR for all patients was 1(1-2) cycles, and 55.56% of patients achieved sustained remission. Seventeen patients had minimal residual disease(MRD) results, with a negative conversion rate of 58.82% for MRD. 33.33% of patients with sustained negative MRD achieved a durable and deep remission. Among the 27 patients, 6 patients(22.22%) had disease progression. The median PFS and OS were not achieved, with a 1-year OS rate of 84.21%. Safety analysis showed that hematologic adverse events were the most common, followed by gastrointestinal symptoms, electrolyte disorders, pulmonary infections, and liver function abnormalities.Conclusion A long course of venetoclax for AML has good efficacy and safety, and some patients can achieve deep and durable remission. Venetoclax can be used as an option for AML patients to consolidate their maintenance therapy.
-
Key words:
- venetoclax /
- acute myeloid leukemia /
- long course of treatment /
- efficacy /
- safety
-

-
表 1 患者基线临床特征
例(%) 临床特征 全部患者(27例) 持续缓解患者(15例) P 性别 1.000 男 13(48.15) 7(46.67) 女 14(51.85) 8(53.33) 年龄 1.000 < 65岁 11(40.74) 6(40.00) ≥65岁 16(59.26) 9(60.00) 初诊WBC 1.000 < 30×109/L 24(88.89) 13(86.67) ≥30×109/L 3(11.11) 2(13.33) 初诊骨髓原始细胞 0.695 < 50% 12(44.44) 7(46.67) ≥50% 13(48.15) 6(40.00) 不详 2(7.41) 2(13.33) 有无合并症 0.424 无 10(37.04) 7(46.67) 有 17(62.96) 8(53.33) AML类型 0.444 原发 26(96.30) 15(100.00) 继发 1(3.70) 0 治疗类型 0.222 初治unfit 14(51.85) 6(40.00) R/R AML 7(25.93) 4(26.67) 巩固治疗 6(22.22) 5(33.33) ELN预后分层 0.071 良好 3(11.12) 2(13.33) 中等 11(40.74) 9(60.00) 不良 7(25.93) 2(13.33) 不详 6(22.22) 2(13.33) 用药强度 0.448 标准/应用唑类减量 18(66.67) 11(73.33) 非唑类减量 9(33.33) 4(26.67) FAB分型 0.719 M0 1(3.70) 1(6.67) M1 1(3.70) 1(6.67) M2 9(33.33) 5(33.33) M4 3(11.12) 2(13.33) M5 5(18.52) 3(20.00) 不详 8(29.63) 3(20.00) 表 2 患者基因突变类型
例(%) 基因突变类型 具有分子遗传学患者(21例) 持续缓解患者(13例) IDH1/2 5(23.81) 3(23.08) NPM1 7(33.33) 5(38.46) DNMT3A 5(23.81) 3(23.08) STAG2 3(14.29) 1(7.69) BCOR 2(9.52) 1(7.69) PTPN11 2(9.52) 2(15.38) FLT3 2(9.52) 1(7.69) FLT3-ITD 2(9.52) 2(15.38) FLT3-TKD 2(9.52) 1(7.69) RUNX1 3(14.29) 1(7.69) CEBPA 2(9.52) 2(15.38) GATA2 2(9.52) 1(7.69) ATM 2(9.52) 1(7.69) WT1 3(14.29) 2(15.38) SRSF2 1(4.76) 0 BCORL1 1(4.76) 0 CREBBP 1(4.76) 1(7.69) IKZF 1(4.76) 1(7.69) TP53 1(4.76) 0 JAK2 1(4.76) 1(7.69) DDX41 1(4.76) 0 CSF3R 1(4.76) 0 MPL 1(4.76) 1(7.69) NRAS 1(4.76) 0 ASXL1 1(4.76) 0 KIT 1(4.76) 0 ZRSR2 1(4.76) 0 SF3B1 1(4.76) 0 AML1-ETO融合基因阳性 1(4.76) 0 融合CBFβ∷MYH11阳性 1(4.76) 0 EVI1 1(4.76) 1(7.69) 表 3 6例疾病进展患者的临床特征
编号 性别 年龄/岁 AML类型 合并症 治疗类型 预后分层 基因突变 首次方案 进展疗程/个 生存状态 1 女 64 原发 有 巩固 不良 IDH1、NPM1、SRSF2 VEN+DEC 10 存活 2 女 58 原发 有 R/R 不详 - VEN+AZA 3 存活 3 女 82 原发 有 初治unfit 不详 - VEN+AZA 中断治疗进展 存活 4 男 67 原发 无 初治unfit 不良 TP53、STAG2、DDX41 VEN+AZA 4 死亡 5 男 76 继发 有 初治unfit 不良 FLT3、ASXL1、KIT、ZRSR2、ATM、RUNX1 VEN+AZA 4 死亡 6 男 52 原发 无 R/R 不良 NRAS、SF3B1、GATA2 VEN+AZA 2 死亡 表 4 影响OS及PFS的单因素预后分析
临床特征 例数 OS PFS χ2 P χ2 P 性别 4.019 0.045 3.340 0.068 男 13 女 14 年龄 0.093 0.760 0.029 0.865 < 65岁 11 ≥65岁 16 初诊WBC 0.455 0.500 0.404 0.525 < 30×109/L 24 ≥30×109/L 3 初诊骨髓原始细胞 3.774 0.052 3.372 0.066 < 50% 12 ≥50% 13 有无合并症 1.192 0.275 1.147 0.284 无 10 有 17 AML类型 10.440 0.001 11.501 0.001 原发 26 继发 1 用药强度 0.004 0.998 0.015 0.992 标准 9 应用唑类减量 9 非唑类减量 9 治疗类型 1.104 0.576 0.968 0.616 初治unfit 14 R/R AML 7 巩固治疗 6 ELN危险分层 9.081 0.011 7.067 0.029 良好 3 中等 11 不良 7 不详 6 -
[1] DiNardo CD, Erba HP, Freeman SD, et al. Acute myeloid leukaemia[J]. Lancet, 2023, 401(10393): 2073-2086. doi: 10.1016/S0140-6736(23)00108-3
[2] 龚敏, 吴迪, 李秋柏, 等. 维奈克拉治疗急性髓系白血病的疗效与安全性的单中心真实世界研究[J]. 临床血液学杂志, 2022, 35(5): 359-363. doi: 10.13201/j.issn.1004-2806.2022.05.012
[3] Khoury JD, Solary E, Abla O, et al. The 5th edition of the world health organization classification of haematolymphoid tumours: Myeloid and histiocytic/dendritic neoplasms[J]. Leukemia, 2022, 36(7): 1703-1719. doi: 10.1038/s41375-022-01613-1
[4] Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with>30% blasts[J]. Blood, 2015, 126(3): 291-299. doi: 10.1182/blood-2015-01-621664
[5] Ferrara F, Barosi G, Venditti A, et al. Consensus-based definition of unfitness to intensive and non-intensive chemotherapy in acute myeloid leukemia: A project of SIE, SIES and GITMO group on a new tool for therapy decision making[J]. Leukemia, 2013, 27(5): 997-999. doi: 10.1038/leu.2012.303
[6] Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN[J]. Blood, 2022, 140(12): 1345-1377. doi: 10.1182/blood.2022016867
[7] Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the acute leukaemias. French-American-british(FAB)co-operative group[J]. Br J Haematol, 1976, 33(4): 451-458. doi: 10.1111/j.1365-2141.1976.tb03563.x
[8] Cairo MS, Bishop M. Tumour lysis syndrome: New therapeutic strategies and classification[J]. Br J Haematol, 2004, 127(1): 3-11. doi: 10.1111/j.1365-2141.2004.05094.x
[9] Griffioen MS, De Leeuw DC, Janssen JJWM, et al. Targeting acute myeloid leukemia with venetoclax; biomarkers for sensitivity and rationale for venetoclax-based combination therapies[J]. Cancers, 2022, 14(14): 3456. doi: 10.3390/cancers14143456
[10] DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia[J]. Blood, 2019, 133(1): 7-17. doi: 10.1182/blood-2018-08-868752
[11] Pratz KW, Jonas BA, Pullarkat V, et al. Long-term follow-up of VIALE-A: Venetoclax and azacitidine in chemotherapy-ineligible untreated acute myeloid leukemia[J]. Am J Hematol, 2024, 99(4): 615-624. doi: 10.1002/ajh.27246
[12] Ivanov V, Yeh SP, Mayer J, et al. Design of the VIALE-M phase Ⅲ trial of venetoclax and oral azacitidine maintenance therapy in acute myeloid leukemia[J]. Future Oncol, 2022, 18(26): 2879-2889. doi: 10.2217/fon-2022-0450
[13] Wei Y, Cao Y, Sun R, et al. Targeting bcl-2 proteins in acute myeloid leukemia[J]. Front Oncol, 2020, 10: 584974. doi: 10.3389/fonc.2020.584974
[14] Lagadinou ED, Sach A, Callahan K, et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells[J]. Cell Stem Cell, 2013, 12(3): 329-341. doi: 10.1016/j.stem.2012.12.013
[15] Ionescu F, David JC, Ravichandran A, et al. Hypomethylating agents and venetoclax for acute myeloid leukemia relapsed after hematopoietic stem cell transplant[J]. Clinical Lymphoma Myeloma Leuk, 2024, 24(6): 400-406. doi: 10.1016/j.clml.2024.02.005
[16] Pollyea DA, DiNardo CD, Arellano ML, et al. Impact of venetoclax and azacitidine in treatment-naïve patients with acute myeloid leukemia and IDH1/2 mutations[J]. Clin Cancer Res, 2022, 28(13): 2753-2761. doi: 10.1158/1078-0432.CCR-21-3467
[17] DiNardo CD, Tiong IS, Quaglieri A, et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML[J]. Blood, 2020, 135(11): 791-803. doi: 10.1182/blood.2019003988
[18] Chua CC, Hammond D, Kent A, et al. Treatment-free remission after ceasing venetoclax-based therapy in patients with acute myeloid leukemia[J]. Blood Adv, 2022, 6(13): 3879-3883. doi: 10.1182/bloodadvances.2022007083
-




 下载:
下载: