Efficacy and safety analysis of continuous treatment of acute myeloid leukemia with venetoclax combined with azacitidine in outpatient settings
-
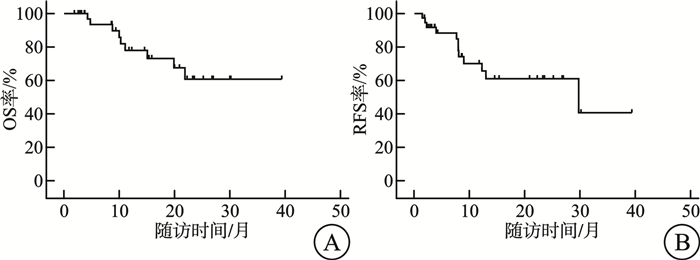
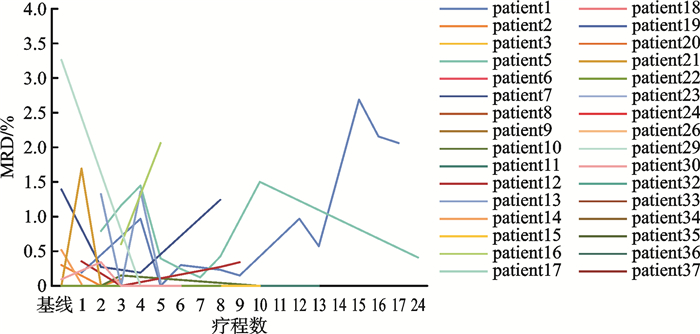
摘要: 目的 评估急性髓系白血病(acute myeloid leukemia,AML)患者缓解后,于门诊接受维奈克拉(venetoclax,VEN)联合阿扎胞苷(azacitidine,AZA)方案(VEN+AZA)持续治疗的疗效、安全性及可行性,为临床实践提供一定的参考。方法 回顾性分析2021年7月—2024年9月于河北医科大学第二医院血液内科门诊接受VEN+AZA持续治疗的AML患者的临床资料。结果 共观察37例患者,中位随访时间为12.3(1.9~39.4)个月,中位疗程数为7(2~29)个疗程,截止随访结束,25例(67.6%)患者获得持续缓解,12例(32.4%)患者出现了复发;在获取微小残留病(minimal residual disease,MRD)结果的32例患者中,23例(71.9%)患者达到最佳MRD阴性,10例(31.3%)患者MRD转阴,且MRD转阴的中位治疗周期为3(2~5)个周期;所有患者的中位总生存期(overall survival,OS)尚未达到,中位无复发生存期(recurrence free survival,RFS)为29.8个月,1年OS率为78.0%,至随访截止为60.7%;1年RFS率为65.4%,至随访截止为40.5%。门诊持续治疗期间最常见的不良事件为血液学不良事件,最常见的非血液学不良事件为肺部感染,所有的不良事件通过积极治疗均得到了控制和耐受,未发生治疗相关性死亡。结论 本中心的真实世界数据表明,AML患者经住院VEN+AZA诱导缓解后,于门诊进行持续治疗具有良好的安全性和可行性,不仅能提升患者生活质量和治疗依从性,还可提高病房周转率。然而,本研究存在一定的局限性,未来仍需开展多中心前瞻性研究,以进一步探究该门诊治疗方案在中国医疗环境中的可行性。Abstract: Objective This study aimed to evaluate the efficacy, safety and feasibility of continuous treatment with the venetoclax(VEN) combined with azacitidine(AZA) regimen(VEN+AZA) in outpatients of our hospital after remission of acute myeloid leukemia(AML), providing certain references for clinical practice.Methods A retrospective analysis was conducted on AML patients who received continuous VEN+AZA treatment in the outpatient department of the Hematology Department of the Second Hospital of Hebei Medical University from July 2021 to September 2024.Results A total of 37 patients were observed. The median follow-up time was 12.3(1.9-39.4) months, and the median number of treatment courses was 7(2-29) courses. By the end of the follow-up, 25 patients(67.6%) achieved continuous remission, and 12 patients(32.4%) relapsed. Among the 32 patients with available minimal residual disease(MRD) results, 23 patients(71.9%) achieved optimal MRD negativity, 10 patients(31.3%) experienced MRD conversion to negative, and the median treatment cycle for MRD conversion to negative was 3(2-5) cycles. The median overall survival(OS) of all patients had not been reached, the median recurrence free survival(RFS) was 29.8 months, the 1-year OS rate was 78.0%, and the OS rate was 60.7% at the end of the follow-up; the 1-year RFS rate was 65.4%, and the RFS rate was 40.5% at the end of the follow-up. The most common adverse events during outpatient continuous treatment were hematological adverse events, and the most common non-hematological adverse event was pulmonary infection. All adverse events were controlled and tolerated through active treatment, and no treatment-related deaths occurred.Conclusion The real-world data of our center indicate that continuous outpatient treatment after in-hospital VEN+AZA induction remission for AML patients has good safety and feasibility. It can not only improve the quality of life and treatment compliance of patients but also increase the ward turnover rate. However, this study has certain limitations, and future multicenter prospective studies are still needed to further explore the feasibility of this outpatient treatment regimen in the Chinese medical environment.
-

-
表 1 患者基因及染色体检测结果
例(%) 类型 数值 基因突变情况 NPM1 13(39.4) FLT3-ITD 9(27.3) IDH1/2 8(24.2) TET2 8(24.2) DNMT3A 6(18.2) CEBPA 5(15.2) ASXL1 4(12.1) TP53 2(6.1) 染色体突变情况 正常核型 21(65.6) 异常核型 11(34.4) 表 2 基因突变及染色体与生存分析的相关性分析
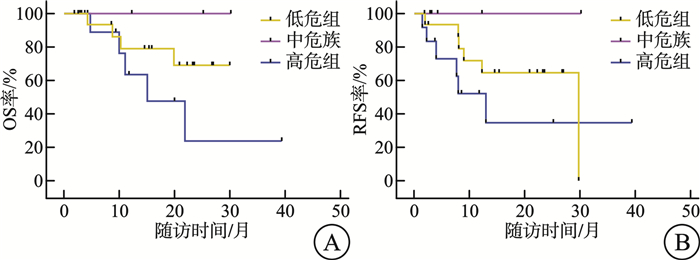
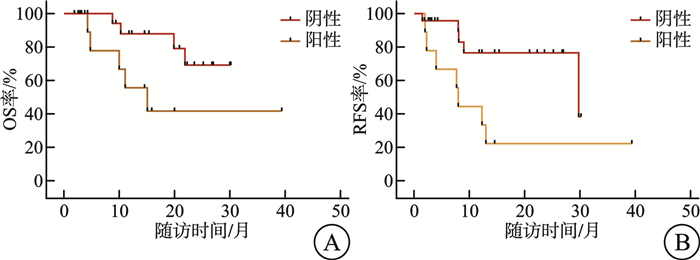
项目 mRFS/月 P mOS/月 P 基因型 NPM1 0.556 0.504 有 12.3 - 无 - - FLT3-ITD 0.009 0.143 有 7.7 11.1 无 - - IDH1/2 0.836 0.239 有 - - 无 13.0 - 染色体核型 0.135 0.470 正常核型 13.0 - 异常核型 - - 注:“-”表示未达到mRFS/mOS。 表 3 门诊VEN+AZA持续治疗不良事件发生率
例(%) 不良事件 无 1级 2级 3级 4级 中性粒细胞减少 263(78.0) 24(7.1) 11(3.3) 23(6.8) 16(4.7) 贫血 299(88.7) 20(5.9) 10(3.0) 4(1.2) 4(1.2) 血小板减少 293(86.9) 13(3.9) 8(2.4) 9(2.7) 14(4.2) 感染 315(93.5) 3(0.9) 4(1.2) 2(0.6) 13(3.9) 胃肠道反应 333(98.8) 2(0.6) 2(0.6) 0 0 电解质紊乱 334(99.1) 2(0.6) 1(0.3) 0 0 注:37例患者于门诊VEN+AZA持续治疗的总疗程数为337次。 -
[1] Medinger M, Heim D, Halter JP, et al. Diagnosis and therapy of acute myeloid leukemia[J]. Ther Umsch, 2019, 76(9): 481-486. doi: 10.1024/0040-5930/a001126
[2] Pollyea DA, Altman JK, Assi R, et al. Acute Myeloid Leukemia, Version 3.2023, NCCN Clinical Practice Guidelines in Oncology[J]. J Natl Compr Canc Netw, 2023, 21(5): 503-513. doi: 10.6004/jnccn.2023.0025
[3] Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN[J]. Blood, 2022, 140(12): 1345-1377. doi: 10.1182/blood.2022016867
[4] 中华医学会血液学分会白血病淋巴瘤学组. 成人急性髓系白血病(非急性早幼粒细胞白血病)中国诊疗指南(2023年版)[J]. 中华血液学杂志, 2023, 44(9): 705-712.
[5] 中国临床肿瘤学会(CSCO)白血病专家委员会. 含维奈克拉方案治疗新诊断不适合强烈化疗急性髓系白血病的专家建议(2024年版)[J]. 白血病·淋巴瘤, 2024, 33(11): 641-648.
[6] Vachhani P, Flahavan EM, Xu T, et al. Venetoclax and Hypomethylating Agents as First-Line Treatment in Newly Diagnosed Patients with AML in a Predominately Community Setting in the US[J]. Oncologist, 2022, 27(11): 907-918. doi: 10.1093/oncolo/oyac135
[7] DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia[J]. N Engl J Med, 2020, 383(7): 617-629. doi: 10.1056/NEJMoa2012971
[8] 中国临床肿瘤学会(CSCO)白血病专家委员会. 维奈克拉治疗恶性血液病临床应用指导原则(2021年版)[J]. 白血病·淋巴瘤, 2021, 30(12): 710-718.
[9] Pratz KW, Jonas BA, Pullarkat V, et al. Long-term follow-up of VIALE-A: Venetoclax and azacitidine in chemotherapy-ineligible untreated acute myeloid leukemia[J]. Am J Hematol, 2024, 99(4): 615-624. doi: 10.1002/ajh.27246
[10] Hu M, Li W, Zhang Y, et al. Venetoclax in adult acute myeloid leukemia[J]. Biomed Pharmacother, 2023, 168: 115820. doi: 10.1016/j.biopha.2023.115820
[11] Patel KK, Zeidan AM, Shallis RM, et al. Cost-effectiveness of azacitidine and venetoclax in unfit patients with previously untreated acute myeloid leukemia[J]. Blood Adv, 2021, 5(4): 994-1002. doi: 10.1182/bloodadvances.2020003902
[12] 赵馨然, 莫璇, 赵瑾, 等. 维奈克拉治疗不适合强诱导化疗的新诊断急性髓系白血病: 真实世界使用以及医疗资源利用情况[J]. 中国药物经济学, 2023, 18(5): 29-36.
[13] 张佳玉, 马晓霖, 赵洪国, 等. 维奈克拉长疗程治疗急性髓系白血病患者的疗效及安全性分析[J]. 临床血液学杂志, 2024, 37(9): 657-662. doi: 10.13201/j.issn.1004-2806.2024.09.011
[14] Ivanov V, Yeh SP, Mayer J, et al. Design of the VIALE-M phase Ⅲ trial of venetoclax and oral azacitidine maintenance therapy in acute myeloid leukemia[J]. Future Oncol, 2022, 18(26): 2879-2889. doi: 10.2217/fon-2022-0450
[15] Morsia E, McCullough K, Joshi M, et al. Venetoclax and hypomethylating agents in acute myeloid leukemia: Mayo Clinic series on 86 patients[J]. Am J Hematol, 2020, 95(12): 1511-1521. doi: 10.1002/ajh.25978
[16] Pratz KW, Jonas BA, Pullarkat V, et al. Measurable Residual Disease Response and Prognosis in Treatment-Naïve Acute Myeloid Leukemia With Venetoclax and Azacitidine[J]. J Clin Oncol, 2022, 40(8): 855-865. doi: 10.1200/JCO.21.01546
[17] 娄典, 刘利, 秦炜炜. 维奈克拉联合阿扎胞苷治疗老年初治急性髓系白血病的临床分析[J]. 中国肿瘤临床, 2022, 49(15): 775-780.
[18] 陈湘, 黄纯兰. 维奈克拉联合阿扎胞苷治疗急性髓系白血病的疗效及复发预测因素分析[J]. 中国实验血液学杂志, 2023, 31(6): 1657-1662.
[19] Gale RE, Green C, Allen C, et al. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia[J]. Blood, 2008, 111(5): 2776-2784. doi: 10.1182/blood-2007-08-109090
[20] Wang QQ, Wang HF, Zhao JZ, et al. Venetoclax for arsenic-resistant acute promyelocytic leukaemia[J]. Br J Haematol, 2022, 197(5): e58-e60.
[21] Li H, Xiang X, Ding H, et al. Differentiation therapy using low-dose venetoclax in a variant acute promyelocytic leukaemia carrying ZBTB16-RARA[J]. Br J Haematol, 2022, 199(5): 768-771. doi: 10.1111/bjh.18476
[22] Wang AY, Weiner H, Green M, et al. A phase I study of selinexor in combination with high-dose cytarabine and mitoxantrone for remission induction in patients with acute myeloid leukemia[J]. J Hematol Oncol, 2018, 11(1): 4. doi: 10.1186/s13045-017-0550-8
[23] Wolach O, Frisch A, Shargian L, et al. Venetoclax in combination with FLAG-IDA-based protocol for patients with acute myeloid leukemia: a real-world analysis[J]. Ann Hematol, 2022, 101(8): 1719-1726. doi: 10.1007/s00277-022-04883-y
[24] Papayannidis C, Nanni J, Cristiano G, et al. Impact of infectious comorbidity and overall time of hospitalization in total outpatient management of acute myeloid leukemia patients following venetoclax and hypomethylating agents[J]. Eur J Haematol, 2022, 108(6): 449-459. doi: 10.1111/ejh.13753
-

计量
- 文章访问数: 307
- 施引文献: 0



 下载:
下载: