Application of next-generation sequencing in the prognosis of higher-risk myelodysplastic neoplasms
-
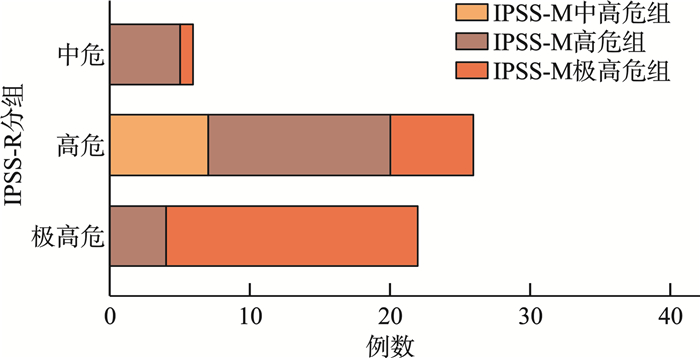
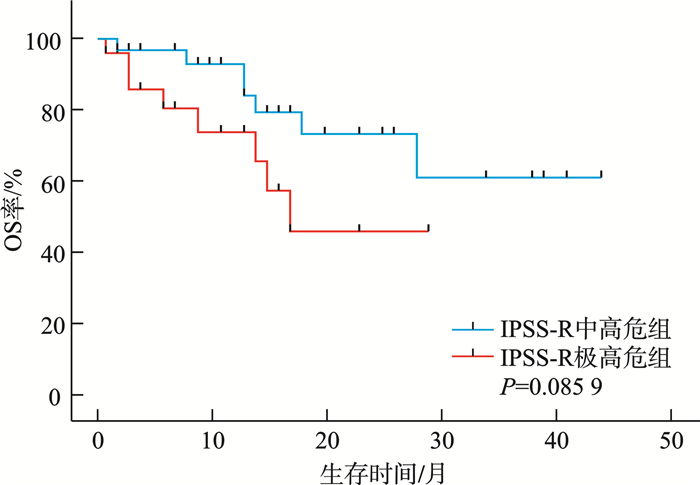
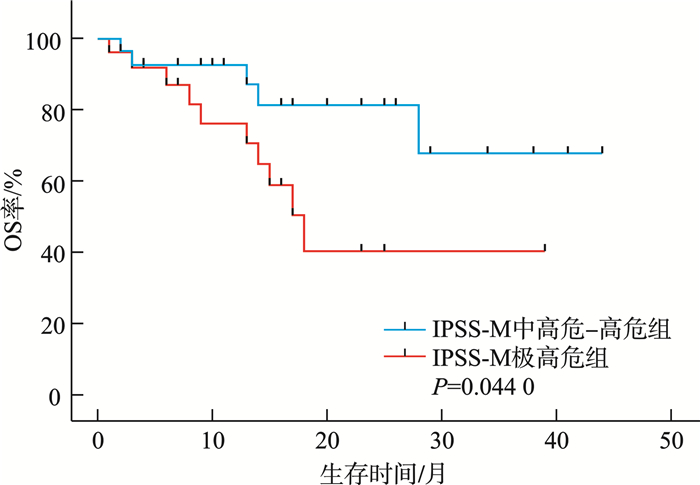
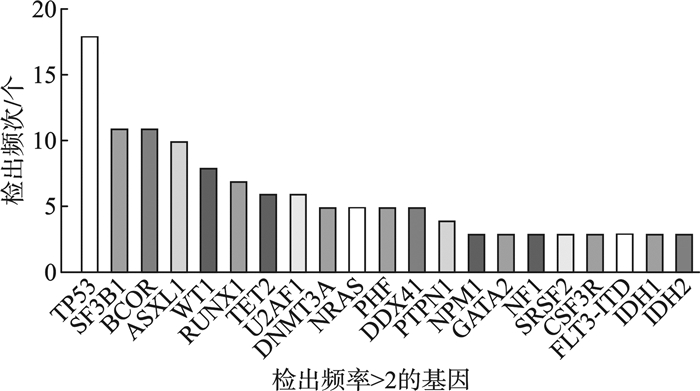
摘要: 目的 分析较高危骨髓增生异常性肿瘤(MDS)的临床和分子遗传学特征,并评价修订的国际预后积分系统(IPSS-R)及MDS分子国际预后评分系统(IPSS-M)对MDS患者的预后价值。方法 纳入中南大学湘雅二医院54例较高危MDS患者,收集患者性别、年龄、临床及实验室检查结果、生存时间。总生存时间(OS)应用Kaplan-Meier曲线分析,组间比较应用log-rank检验。结果 共纳入54例较高危MDS患者,男33例,女21例。中位年龄57(18~87)岁。初诊时中位血红蛋白为69(33~119) g/L,中位中性粒细胞计数为0.64×109/L(0.10×109/L~11.29×109/L),中位血小板计数为45×109/L(8×109/L~395×109/L),中位骨髓原始细胞百分比为9.0%(2.0%~18.0%),11例(20.37%)患者携带复杂核型。54例患者中51例(94.44%)检出至少1个Ⅰ类突变。共有34个突变基因检测到突变总数145个,平均每个患者检测到2.69个突变。最常观察到发生突变的基因是TP53、SF3B1、BCOR、ASXL1、WT1等。TP53基因共在13例患者中检出基因异常。中位随访时间13个月,IPSS-R分组极高危组中位生存时间为17个月,中高危组未达到,差异无统计学意义(P=0.085 9)。IPSS-M分组极高危组中位生存时间为18个月,中高危-高危组未达到,两组1年OS率分别为74%和93%,差异有统计学意义(P=0.044 0)。结论 IPSS-M对本组较高危MDS患者OS具有良好预测能力。建议推进MDS患者进行细胞遗传学及二代测序检查,后续进行多中心、大样本研究,继续验证IPSS-M在MDS预后判断的效力。
-
关键词:
- 骨髓增生异常性肿瘤 /
- 二代测序 /
- MDS分子国际预后评分系统 /
- 修订的国际预后积分系统
Abstract: Objective To analyze the clinical and molecular genetic characteristics of higher-risk myelodysplastic neoplasms(MDS) and to evaluate the prognostic value of the revised international prognostic scoring system(IPSS-R) and the molecular international prognostic scoring system(IPSS-M) for MDS patients.Methods A total of 54 higher-risk MDS patients from the Second Xiangya Hospital of Central South University were included. Data on gender, age, clinical and laboratory test results, and survival time were collected. Overall survival(OS) was analyzed using Kaplan-Meier curves, and intergroup comparisons were performed using the log-rank test.Results Among the 54 higher-risk MDS patients, 33 were male and 21 were female, with a median age of 57(18-87) years. At initial diagnosis, the median hemoglobin level was 69(33-119) g/L, the median absolute neutrophil count was 0.64×109/L(0.10×109/L-11.29×109/L), and the median platelet count was 45×109/L(8×109/L-395×109/L). The median bone marrow blast percentage was 9.0%(2.0%-18.0%), and 11 patients(20.37%) carried complex karyotypes. At least one class Ⅰ mutation was detected in 51 patients(94.44%). A total of 145 mutations across 34 genes were identified, with an average of 2.69 mutations per patient. The most frequently mutated genes included TP53, SF3B1, BCOR, ASXL1 and WT1. TP53 abnormalities were detected in 13 patients. During a median follow-up of 13 months, the median OS in the IPSS-R very high-risk group was 17 months, while it was not reached in the intermediate-high-risk group, with no significant difference(P=0.085 9). In the IPSS-M very high-risk group, the median OS was 18 months, while it was not reached in the intermediate-high-risk to high-risk group. The 1-year survival rates were 74% and 93%, respectively, showing a statistically significant difference(P=0.044 0).Conclusion The IPSS-M prognostic system demonstrated robust predictive capacity for OS in this cohort of higher-risk MDS patients. Based on these findings, we propose routine implementation of cytogenetic analysis and next-generation sequencing in the diagnostic workup of MDS patients. -

-
表 1 较高危MDS患者的临床资料特征
中位数(范围),例(%) 临床特征 数值 年龄/岁 57(18~87) 性别 男 33(61.1) 女 21(38.9) Hb/(g/L) 69(33~119) ANC/(×109/L) 0.64(0.10~11.29) PLT(×109/L) 45(8~395) 骨髓原始细胞/% 9.0(2.0~18.0) 复杂核型 11(20.37) 分型 MDS-biTP53 6(11.11) MDS-骨髓纤维化 1(1.85) MDS-LB 6(11.11) MDS-原始细胞增多型 41(75.93) -
[1] Sekeres MA, Taylor J. Diagnosis and Treatment of Myelodysplastic Syndromes: A Review[J]. JAMA, 2022, 328(9): 872-880. doi: 10.1001/jama.2022.14578
[2] Ogawa S. Genetics of MDS[J]. Blood, 2019, 133(10): 1049-1059. doi: 10.1182/blood-2018-10-844621
[3] Kennedy AL, Shimamura A. Genetic predisposition to MDS: clinical features and clonal evolution[J]. Blood, 2019, 133(10): 1071-1085. doi: 10.1182/blood-2018-10-844662
[4] Bejar R, Stevenson K, Abdel-Wahab O, et al. Clinical effect of point mutations in myelodysplastic syndromes[J]. N Engl J Med, 2011, 364(26): 2496-2506. doi: 10.1056/NEJMoa1013343
[5] Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes[J]. Blood, 2012, 120(12): 2454-2465. doi: 10.1182/blood-2012-03-420489
[6] Haferlach T, Nagata Y, Grossmann V, et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes[J]. Leukemia, 2014, 28(2): 241-247. doi: 10.1038/leu.2013.336
[7] Bernard E, Tuechler H, Greenberg PL, et al. Molecular International Prognostic Scoring System for Myelodysplastic Syndromes[J]. NEJM Evid, 2022, 1(7): EVIDoa2200008.
[8] Hou HA, Tsai CH, Lin CC, et al. Incorporation of mutations in five genes in the revised International Prognostic Scoring System can improve risk stratification in the patients with myelodysplastic syndrome[J]. Blood Cancer J, 2018, 8(4): 39. doi: 10.1038/s41408-018-0074-7
[9] Kewan T, Durmaz A, Bahaj W, et al. Molecular patterns identify distinct subclasses of myeloid neoplasia[J]. Nat Commun, 2023, 14(1): 3136. doi: 10.1038/s41467-023-38515-4
[10] Wu J, Zhang Y, Qin T, et al. IPSS-M has greater survival predictive accuracy compared with IPSS-R in persons ≥ 60 years with myelodysplastic syndromes[J]. Exp Hematol Oncol, 2022, 11(1): 73. doi: 10.1186/s40164-022-00328-4
[11] Huang N, Song Y, Wu L, et al. Validation and improvement of the molecular international prognostic scoring system in Chinese patients with myelodysplastic syndromes[J]. Ann Hematol, 2025, 104(1): 193-206.
[12] Valent P, Horny HP, Bennett JM, et al. Definitions and standards in the diagnosis and treatment of the myelodysplastic syndromes: Consensus statements and report from a working conference[J]. Leuk Res, 2007, 31(6): 727-736. doi: 10.1016/j.leukres.2006.11.009
[13] Valent P, Orazi A, Steensma DP, et al. Proposed minimal diagnostic criteria for myelodysplastic syndromes(MDS)and potential pre-MDS conditions[J]. Oncotarget, 2017, 8(43): 73483-73500. doi: 10.18632/oncotarget.19008
[14] Khoury JD, Solary E, Abla O, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms[J]. Leukemia, 2022, 36(7): 1703-1719. doi: 10.1038/s41375-022-01613-1
[15] Grob T, Al Hinai ASA, Sanders MA, et al. Molecular characterization of mutant TP53 acute myeloid leukemia and high-risk myelodysplastic syndrome[J]. Blood, 2022, 139(15): 2347-2354. doi: 10.1182/blood.2021014472
[16] Kaisrlikova M, Vesela J, Kundrat D, et al. RUNX1 mutations contribute to the progression of MDS due to disruption of antitumor cellular defense: a study on patients with lower-risk MDS[J]. Leukemia, 2022, 36(7): 1898-1906. doi: 10.1038/s41375-022-01584-3
[17] Welch JS, Petti AA, Miller CA, et al. TP53 and Decitabine in Acute Myeloid Leukemia and Myelodysplastic Syndromes[J]. 2016, 375(21): 2023-2036.
[18] Lontos K, Saliba RM, Kanagal-Shamanna R, et al. TP53 Mutant Variant Allele Frequency and Cytogenetics Determine Prognostic Groups in MDS/AML for Transplantation[J]. Blood Adv, 2025: bloodadvances. 2024014499.
[19] Senapati J, Loghavi S, Garcia-Manero G, et al. Clinical interrogation of TP53 aberrations and its impact on survival in patients with myeloid neoplasms[J]. Haematologica, 2024.
[20] Wang J, Li S, Jiang H, et al. Sintilimab plus decitabine for higher-risk treatment-naïve myelodysplastic syndromes: efficacy, safety, and biomarker analysis of a phase Ⅱ, single-arm trial[J]. J Immunother Cancer, 2024, 12(11): e010355. doi: 10.1136/jitc-2024-010355
[21] Sauta E, Robin M, Bersanelli M, et al. Real-World Validation of Molecular International Prognostic Scoring System for Myelodysplastic Syndromes[J]. J Clin Oncol, 2023, 41(15): 2827-2842. doi: 10.1200/JCO.22.01784
[22] Gurnari C, Gagelmann N, Badbaran A, et al. Outcome prediction in myelodysplastic neoplasm undergoing hematopoietic cell transplant in the molecular era of IPSS-M[J]. Leukemia, 2023, 37(3): 717-719. doi: 10.1038/s41375-023-01820-4
[23] Baer C, Huber S, Hutter S, et al. Risk prediction in MDS: independent validation of the IPSS-M-ready for routine?[J]. Leukemia, 2023, 37(4): 938-941. doi: 10.1038/s41375-023-01831-1
[24] Aguirre LE, Al Ali N, Sallman DA, et al. Assessment and validation of the molecular international prognostic scoring system for myelodysplastic syndromes[J]. Leukemia, 2023, 37(7): 1530-1539. doi: 10.1038/s41375-023-01910-3
-

计量
- 文章访问数: 75
- 施引文献: 0



 下载:
下载: