Analysis of clinical characteristics and prognosis of 288 patients with follicular lymphoma
-
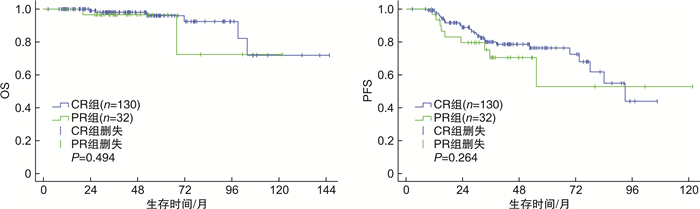
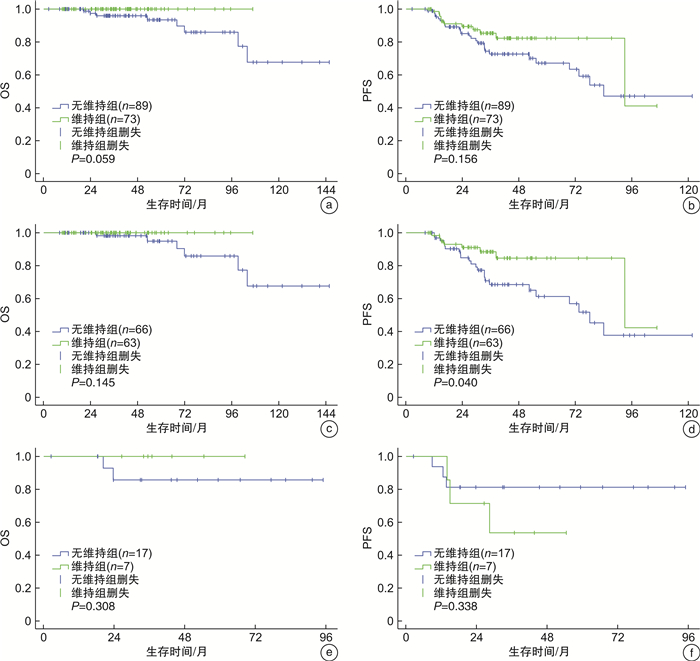
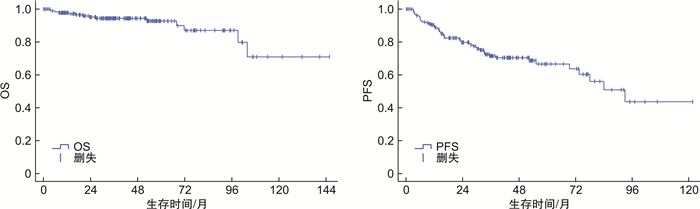
摘要: 目的 探讨滤泡性淋巴瘤(FL)患者的临床特点及预后相关因素。方法 回顾性分析2009年1月—2020年6月复旦大学附属中山医院血液科住院治疗的288例初诊FL患者的临床资料,分析其临床特点、生存及预后因素。结果 ① 中位年龄55岁,男141例,女147例。FL 1~2级占58.3%,FL 3A级占24.7%,FL 3B级占3.5%,复合FL占9.7%,其他(分级不明)占3.8%。83.7%住院治疗FL患者处于Ⅲ~Ⅳ期,41.7%骨髓受侵。②系统治疗总有效率为89.7%,完全缓解(CR)率为70.4%;24.1%患者2年内复发进展。③R-CHOP样组生存分析显示,3年总生存(OS)率为94.4%,3年无进展生存(PFS)率为71.5%;FL 1~3A级患者,终末疗效CR组和部分缓解(PR)组3年OS率分别为98.7%和96.3%(P=0.935),3年PFS率分别为79.4%和70.5%(P=0.284);FL 1~3A级患者,维持治疗组和非维持治疗组3年OS率分别为100.0%和98.1%(P=0.145),3年PFS率分别为88.4%和68.5%(P=0.040);FL 3B级及复合FL患者,维持治疗组和非维持治疗组3年OS率分别为100.0%和85.7%(P=0.308),3年PFS率分别为53.6%和81.3%(P=0.338)。④R-CHOP样组多因素分析发现,FL 3B级及复合FL(P< 0.001)和ECOG评分≥1分(P=0.005)是OS的独立危险因素;ECOG评分≥1分是PFS的独立危险因素(P=0.022)。结论 FL多见于中老年人,接受R-CHOP样方案患者总体预后好。FL 1~3A级患者,终末疗效达CR和PR生存差异无统计学意义,维持治疗PFS获益,OS无明显获益;而FL 3B级和复合FL患者维持治疗可能无明显获益。Abstract: Objective To investigate the clinical characteristics and prognostic factors of patients with follicular lymphoma(FL).Methods We retrospectively analyzed the clinical characteristics, survival and prognostic factors of 288 newly diagnosed FL patients hospitalized at the Department of Hematology in Zhongshan Hospital Affiliated Fudan University between January, 2009 and June, 2020.Results ① Median age was 55 years with 141 males and 147 females. FL grade 1-2 accounted for 58.3%, grade 3A accounted for 24.7%, grade 3B accounted for 3.5%, composite FL accounted for 9.7% and unknown grade accounted for 3.8%. Ann Arbor stage Ⅲ-Ⅳ accounted for 83.7% of hospitalized FL patients and 41.7% patients had bone marrow involvement. ②The overall response rate was 89.7%, complete remission(CR) rate was 70.4%; progression of disease in the first two years accounted for 24.1%. ③In R-CHOP group, the 3-year overall survival(OS) rate was 94.4%, the 3-year progression-free survival(PFS) rate was 71.5%. Among FL grade 1-3A patients, the 3-year OS rates of CR group and partial remission(PR) group at the end of treatment were 98.7% and 96.3%(P=0.935), the 3-year PFS rates were 79.4% and 70.5%(P=0.284). Among FL grade 1-3A patients, the 3-year OS rates of the maintenance group and non-maintenance group were 100.0% and 98.1%(P=0.145), the 3-year PFS rates were 88.4% and 68.5%(P=0.040). For FL grade 3B and composite FL patients, the 3-year OS rates of the maintenance group and non-maintenance group were 100.0% and 85.7%(P=0.308), the 3-year PFS rates were 53.6% and 81.3%(P=0.338). ④Multivariate analysis showed that FL grade 3B and composite FL(P< 0.001) and ECOG score≥1(P=0.005) were independent risk factors of OS. ECOG score≥1 was an independent risk factor of PFS(P=0.022).Conclusion FL is more common among middle-aged and elderly people and overall prognosis is promising. Among FL grade 1-3A patients, there are no significant survival difference between CR and PR patients at the end of frontline immunochemotherapy. Maintenance therapy brings benefits for FL grade 1-3A patients on PFS but not OS. FL grade 3B and composite FL patients probably couldn't benefit from maintenance therapy.
-
Key words:
- follicular lymphoma /
- clinical characteristics /
- survival /
- maintenance
-

-
表 1 288例FL患者的基线特征
例(%) 临床特征 总体(288例) 系统治疗组(242例) 观察等待组(40例) 放疗组(6例) 中位年龄(范围)/岁 55(21~85) 54(21~84) 55(34~85) 65(37~80) 男性 141(49.0) 108(44.6) 28(70.0) 5(83.3) 年龄≥ 60岁 108(37.5) 85(35.1) 19(47.5) 4(66.7) 组织学分级 1~2级 168(58.3) 126(52.1) 37(92.5) 5(83.3) 3A级 71(24.7) 68(28.1) 3(7.5) 0 3B级 10(3.5) 10(4.1) 0 0 FL合并DLBCL 28(9.7) 27(11.2) 0 1(16.7) 其他 11(3.8) 11(4.5) 0 0 Ann Arbor Ⅲ~Ⅳ期 241(83.7) 204(84.3) 36(90.0) 1(16.7) ECOG评分≥2分 48(16.7) 44(18.2) 3(7.5) 1(16.7) 合并B症状 75(26.0) 73(30.2) 2(5.0) 0 血红蛋白 < 120 g/L 77(26.7) 69(28.5) 6(15.0) 2(33.3) LDH升高 57(19.8) 53(21.9) 4(10.0) 0 血β2-MG升高 154(53.5) 143(59.1) 10(25.0) 1(16.7) HBs-Ag阳性 36(12.5) 33(13.6) 3(7.5) 0 骨髓受侵 120(41.7) 109(45.0) 11(27.5) 0 淋巴结区域>4 193(67.0) 165(68.2) 26(65.0) 2(33.3) 大肿物(直径>6 cm) 83(28.8) 83(34.3) 0 0 结外器官受侵≥2 78(27.1) 76(31.4) 2(5.0) 0 FLIPI(273例) 低-中危(0~2分) 153(56.0) 128(55.4) 21(58.3) 4(66.7) 高危(3~5分) 120(44.0) 103(44.6) 15(41.7) 2(33.3) FLIPI-2(269例) 低-中危(0~2分) 166(61.7) 133(57.8) 28(84.8) 5(83.3) 高危(3~5分) 103(38.3) 97(42.2) 5(15.2) 1(16.7) PRIMA预后指数(268例) 低-中危(0~1分) 182(67.9) 149(64.8) 29(87.9) 4(80.0) 高危(2分) 86(32.1) 81(35.2) 4(12.1) 1(20.0) 表 2 242例患者的一线治疗方案和终末疗效评估
例(%) 一线治疗方案 可评估的例数/总例数 ORR CR PR SD+PD “无化疗”方案 利妥昔单抗单药 2/2 2(100.0) 2(100.0) 0 0 利妥昔单抗+来那度胺 21/22 19(90.5) 10(47.6) 9(42.9) 2(9.5) 免疫化疗 利妥昔单抗+CHOP样方案 177/184 162(91.5) 130(73.4) 32(18.1) 15(8.5) 利妥昔单抗+苯达莫司汀 5/5 5(100.0) 5(100.0) 0 0 利妥昔单抗+氟达拉滨+环磷酰胺/米托蒽醌+泼尼松 6/6 4(66.7) 4(66.7) 0 2(33.3) 利妥昔单抗+克拉屈滨 4/4 4(100.0) 3(75.0) 1(25.0) 0 单纯化疗 CHOP样方案 17/18 12(70.6) 9(52.9) 3(17.7) 5(29.4) 氟达拉滨+环磷酰胺 1/1 1(100.0) 1(100.0) 0 0 合计 233/242 209(89.7) 164(70.4) 45(19.3) 24(10.3) 表 3 184例R-CHOP样组患者预后的单因素分析
变量 3年PFS率/% P 3年OS率/% P 组织分级 0.053 < 0.001 1~3A级 72.4 99.0 3B级+复合FL 61.9 77.1 ECOG评分 0.014 0.043 ≥1分 63.5 91.6 0分 78.1 96.4 B症状 0.320 0.088 有 69.1 89.8 无 72.2 96.2 血红蛋白 0.091 0.011 < 120 g/L 65.2 86.5 ≥120 g/L 76.0 97.3 LDH 0.398 0.002 升高 72.1 84.1 正常 73.0 97.4 β2-MG 0.244 0.047 升高 72.5 92.0 正常 72.2 97.9 骨髓受侵 0.022 0.310 是 63.2 93.4 否 80.6 95.0 受累淋巴结区域>4 0.014 0.485 是 65.4 93.9 否 78.1 95.6 结外器官受侵≥2 0.017 0.135 是 61.4 91.0 否 76.3 96.2 表 4 184例R-CHOP样组患者预后的多因素分析
变量 PFS OS HR(95%CI) P HR(95%CI) P FL 3B级+复合FL 23.40(5.05~108.31) < 0.001 ECOG评分≥1分 2.01(1.11~3.65) 0.022 8.34(1.88~36.96) 0.005 -
[1] Teras LR, Desantis CE, Cerhan JR, et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes[J]. CA Cancer J Clin, 2016, 66(6): 443-459. doi: 10.3322/caac.21357
[2] 李小秋, 李甘地, 高子芬, 等. 中国淋巴瘤亚型分布: 国内多中心性病例10002例分析[J]. 诊断学理论与实践, 2012, 11(2): 111-115. https://www.cnki.com.cn/Article/CJFDTOTAL-ZDLS201202007.htm
[3] 周立强, 李晔雄, 孙云田, 等. 非霍奇金淋巴瘤1125例临床病理分析[J]. 癌症进展, 2006, 4(5): 391-397. doi: 10.3969/j.issn.1672-1535.2006.05.004
[4] Batlevi CL, Sha F, Alperovich A, et al. Follicular lymphoma in the modern era: survival, treatment outcomes, and identification of high-risk subgroups[J]. Blood Cancer J, 2020, 10(7): 74. doi: 10.1038/s41408-020-00340-z
[5] Engelhard M. Anti-CD20 antibody treatment of non-Hodgkin lymphomas[J]. Clin Immunol, 2016, 172: 101-104. doi: 10.1016/j.clim.2016.08.011
[6] Provencio M, Sabin P, Gomez-Codina J, et al. Impact of treatment in long-term survival patients with follicular lymphoma: A Spanish Lymphoma Oncology Group registry[J]. PLoS One, 2017, 12(5): e0177204. doi: 10.1371/journal.pone.0177204
[7] Casulo C, Byrtek M, Dawson KL, et al. Early Relapse of Follicular Lymphoma After Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone Defines Patients at High Risk for Death: An Analysis From the National LymphoCare Study[J]. J Clin Oncol, 2015, 33(23): 2516-2522. doi: 10.1200/JCO.2014.59.7534
[8] Al-Tourah AJ, Gill KK, Chhanabhai M, et al. Population-based analysis of incidence and outcome of transformed non-Hodgkin's lymphoma[J]. J Clin Oncol, 2008, 26(32): 5165-5169. doi: 10.1200/JCO.2008.16.0283
[9] Wagner-Johnston ND, Link BK, Byrtek M, et al. Outcomes of transformed follicular lymphoma in the modern era: a report from the National LymphoCare Study(NLCS)[J]. Blood, 2015, 126(7): 851-857. doi: 10.1182/blood-2015-01-621375
[10] Ambinder AJ, Shenoy PJ, Malik N, et al. Exploring risk factors for follicular lymphoma[J]. Adv Hematol, 2012, 2012: 626035.
[11] Sabattini E, Bacci F, Sagramoso C, et al. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview[J]. Pathologica, 2010, 102(3): 83-87.
[12] Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification[J]. J Clin Oncol, 2014, 32(27): 3059-3068. doi: 10.1200/JCO.2013.54.8800
[13] Junlen HR, Peterson S, Kimby E, et al. Follicular lymphoma in Sweden: nationwide improved survival in the rituximab era, particularly in elderly women: a Swedish Lymphoma Registry study[J]. Leukemia, 2015, 29(3): 668-676. doi: 10.1038/leu.2014.251
[14] 王楠, 许彭鹏, 王黎, 等. 利妥昔单抗联合化疗治疗229例滤泡性淋巴瘤患者的预后研究[J]. 中华血液学杂志, 2019, 40(1): 46-51.
[15] 吕柯冰, 李鑫, 左伟莉, 等. 不同年龄组低级别滤泡淋巴瘤的临床特征及预后分析[J]. 中国肿瘤临床, 2020, 47(16): 811-816. doi: 10.3969/j.issn.1000-8179.2020.16.804
[16] Barrington SF, Mikhaeel NG, Kostakoglu L, et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group[J]. J Clin Oncol, 2014, 32(27): 3048-3058. doi: 10.1200/JCO.2013.53.5229
[17] Gallamini A, Borra A. FDG-PET Scan: a new Paradigm for Follicular Lymphoma Management[J]. Mediterr J Hematol Infect Dis, 2017, 9(1): e2017029. doi: 10.4084/mjhid.2017.029
[18] 孙悦, 许宏, 郭振清, 等. 探索18F-FDG PET/CT SUVmax, SUVsum及病理Ki67表达等在非霍奇金淋巴瘤中的临床应用价值[J]. 临床血液学杂志, 2021, 34(1): 18-23. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXZ202101005.htm
[19] Fisher RI, Leblanc M, Press OW, et al. New treatment options have changed the survival of patients with follicular lymphoma[J]. J Clin Oncol, 2005, 23(33): 8447-8452. doi: 10.1200/JCO.2005.03.1674
[20] Rajai H, Bodor C, Balogh Z, et al. Impact of the reactive microenvironment on the bone marrow involvement of follicular lymphoma[J]. Histopathology, 2012, 60(6B): E66-E75. doi: 10.1111/j.1365-2559.2012.04187.x
[21] Sorigue M, Sancho JM. Current prognostic and predictive factors in follicular lymphoma[J]. Ann Hematol, 2018, 97(2): 209-227. doi: 10.1007/s00277-017-3154-z
[22] Lopci E, Zanoni L, Chiti A, et al. FDG PET/CT predictive role in follicular lymphoma[J]. Eur J Nucl Med Mol Imaging, 2012, 39(5): 864-871. doi: 10.1007/s00259-012-2079-y
[23] 卢可, 韩雪, 张会来. 早期复发进展滤泡性淋巴瘤的预后及危险因素分析[J]. 中国肿瘤临床, 2020, 47(7): 344-349. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGZL202007006.htm
[24] Bachy E, Brice P, Delarue R, et al. Long-term follow-up of patients with newly diagnosed follicular lymphoma in the prerituximab era: effect of response quality on survival-A study from the groupe d'etude des lymphomes de l'adulte[J]. J Clin Oncol, 2010, 28(5): 822-829. doi: 10.1200/JCO.2009.22.7819
[25] Bachy E, Seymour JF, Feugier P, et al. Sustained Progression-Free Survival Benefit of Rituximab Maintenance in Patients With Follicular Lymphoma: Long-Term Results of the PRIMA Study[J]. J Clin Oncol, 2019, 37(31): 2815-2824. doi: 10.1200/JCO.19.01073
[26] Zhou Y, Qin Y, He X, et al. Long-term survival and prognostic analysis of advanced stage follicular lymphoma in the rituximab era: A China single-center retrospective study[J]. Asia Pac J Clin Oncol, 2021, 17(3): 289-299.
-




 下载:
下载: