Efficacy and safety of bendamustine plus rituximab in Chinese de novo margin zone lymphoma patients: A multicenter retrospective study based on propensity score matching
-
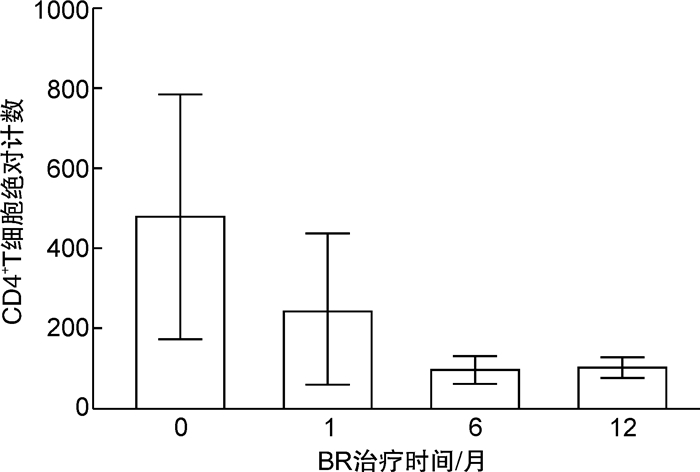
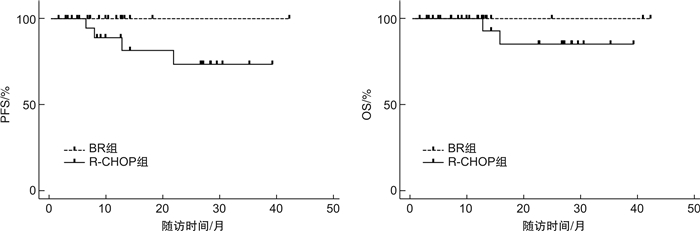
摘要: 目的 分析苯达莫司汀联合利妥昔单抗(BR)方案在边缘区B细胞淋巴瘤(MZL)患者中的疗效和安全性。方法 回顾性分析2018年1月1日—2020年12月31日在上海和青岛的4家医院接受BR方案作为一线治疗的20例初治MZL患者的临床特点、疗效及治疗相关不良事件,应用倾向性评分匹配(PSM)将接受利妥昔单抗联合环磷酰胺、阿霉素、长春新碱和泼尼松(R-CHOP)方案治疗的20例初治MZL患者作为对照组,对比2组疗效和安全性。结果 BR组总反应率为90.0%,完全缓解率为60.0%;中位随访时间11.7(5.2~42.3)个月,1年无进展生存率和1年总生存率均为100.0%;最常见的血液学不良反应为CD4阳性淋巴细胞减少(13例)和粒细胞减少(5例),常见的非血液学不良反应为感染性发热(4例)和纳差(3例)。BR组和R-CHOP组的总反应率(90.0% vs 95.0%,P=1.000)、完全缓解率(60.0% vs 60.0%,P=1.000)比较差异均无统计学意义。R-CHOP组粒细胞减少(60.0% vs 25.0%,P=0.054)、脱发(85.0% vs 0,P< 0.001)、恶心呕吐(75.0% vs 0,P< 0.001)和乏力(40.0% vs 5.0%,P=0.020)不良反应的发生率显著高于BR组,其他不良反应2组间比较差异无统计学意义。结论 BR方案治疗MZL有效且安全。Abstract: Objective To evaluate the efficacy and safety of bendamustine plus rituximab(BR) regimen in de novo margin zone lymphoma(MZL) patients.Methods Clinical records of 20 de novo MZL patients received BR regimen in 4 hospitals in Shanghai and Qingdao from January 1st, 2018 to December 31st, 2020 were selected. Using propensity score matching(PSM)(1∶1 matching), 20 de novo MZL patients treated with rituximab combined with cyclophosphamide, doxorubicin, vincristine and prednisone(R-CHOP) were collected as the control group. The clinical characters, efficacy and safety between BR group and R-CHOP group were analyzed.Results In BR group, the overall response rate was 90.0% and the complete response rate was 60.0%. With a median follow-up of 11.7 months(5.2-42.3 months), the 1-year progression free survival rate and 1-year overall response rate were 100.0%. The commom hematological adverse events were decreased CD4+lymphocytes(13 cases) and neutropenia(5 cases). The common non-hematological adverse events were fever(4 cases) and anorexia(3 cases). Compared with R-CHOP group, there was no significant difference in overall response rate(90.0% vs 95.0%,P=1.000) and complete response rate(60.0% vs 60.0%,P=1.000). Patients in R-CHOP group suffered significantly higher rate of neutropenia(60.0% vs 25.0%,P=0.054), alopecia(85.0% vs 0,P< 0.001), nausea/vomiting(75.0% vs 0,P< 0.001) and fatigue(40.0% vs 5.0%,P=0.020).Conclusion BR regimen is effective and safe for de novo MZL patients.
-
Key words:
- bendamustine /
- rituximab /
- margin zone lymphoma /
- efficacy /
- safety /
- propensity score matching
-

-
表 1 BR组和R-CHOP组临床基线特征
例(%) 临床特征 PSM前 PSM后 BR组(20例) R-CHOP组
(94例)P BR组(20例) R-CHOP组
(20例)P 男性 10(50.0) 55(58.5) 0.485 10(50.0) 11(55.0) 0.752 年龄≥70岁 5(25.0) 12(12.8) 0.175 5(25.0) 3(15.0) 0.695 病理亚型 0.406 0.493 MALT 18(90.0) 77(81.9) 18(90.0) 19(95.0) NZML 1(5.0) 14(14.9) 1(5.0) 1(5.0) SMZL 1(5.0) 3(3.2) 1(5.0) 0 Ann Arbor Ⅲ/Ⅳ期 13(65.0) 66(70.2) 0.646 13(65.0) 11(55.0) 0.519 ECOG≥2 9(45.0) 30(31.9) 0.263 9(45.0) 7(35.0) 0.519 B症状 12(60.0) 31(33.0) 0.024 12(60.0) 10(50.0) 0.525 MALT-IPI 0.025 0.233 低危 12(60.0) 73(77.7) 12(60.0) 15(75.0) 中危 6(30.0) 21(22.3) 6(30.0) 5(25.0) 高危 2(10.0) 0 2(10.0) 0 ki67 0.937 0.179 ≤15% 13(65.0) 60(63.8) 13(65.0) 17(85.0) 15%<ki67≤30% 5(25.0) 22(23.4) 5(25.0) 1(5.0) >30% 2(10.0) 12(12.8) 2(10.0) 2(10.0) 血红蛋白 < 120 g/L 7(35.0) 14(14.9) 0.035 7(35.0) 5(25.0) 0.490 血小板减少 2(10.0) 5(5.3) 0.604 2(10.0) 1(5.0) 1.000 LDH升高 5(25.0) 14(14.9) 0.321 5(25.0) 4(20.0) 1.000 β2-MG升高 12(60.0) 36(38.3) 0.074 12(60.0) 8(40.0) 0.206 D-二聚体升高 3(15.0) 23(24.5) 0.558 3(15.0) 5(25.0) 0.695 骨髓累及 6(30.0) 19(20.2) 0.376 6(30.0) 4(20.0) 0.465 淋巴结受累个数≥6 4(20.0) 27(28.7) 0.426 4(20.0) 2(10.0) 0.661 结外受累器官≥2 4(20.0) 17(18.1) 0.761 4(20.0) 3(15.0) 1.000 表 2 BR组和R-CHOP组疗效和预后
疗效 BR组
(20例)R-CHOP组
(20例)P ORR/例(%) 18(90.0) 19(95.0) 1.000 CR/例(%) 12(60.0) 12(60.0) 1.000 1年PFS/% 100.0 88.9 0.487 1年OS/% 100.0 100.0 1.000 1.5年PFS/% 100.0 81.5 0.106 1.5年OS/% 100.0 85.1 0.231 -
[1] Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms[J]. Blood, 2016, 127(20): 2375-2390. doi: 10.1182/blood-2016-01-643569
[2] 张怡安, 魏征, 庄静丽, 等. 利妥昔单抗联合克拉屈滨在惰性B细胞淋巴瘤患者中的疗效及安全性研究[J]. 临床血液学杂志, 2021, 34(7): 489-494. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXZ202107008.htm
[3] Raderer M, Kiesewette RB, Ferreri AJ. Clinicopathologic characteristics and treatment of marginal zone lymphoma of mucosa-associated lymphoid tissue(MALT lymphoma)[J]. CA Cancer J Clin, 2016, 66(2): 153-171.
[4] Nakamura S, Sugiyama T, Matsumoto T, et al. Long-term clinical outcome of gastric MALT lymphoma after eradication of Helicobacter pylori: a multicentre cohort follow-up study of 420 patients in Japan[J]. Gut, 2012, 61(4): 507-513. doi: 10.1136/gutjnl-2011-300495
[5] Stathis A, Chini C, Bertoni F, et al. Long-term outcome following Helicobacter pylori eradication in a retrospective study of 105 patients with localized gastric marginal zone B-cell lymphoma of MALT type[J]. Ann Oncol, 2009, 20(6): 1086-1093. doi: 10.1093/annonc/mdn760
[6] ZuccA E, CopiE-Bergman C, Ricardi U, et al. Gastric marginal zone lymphoma of MALT type: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up[J]. Ann Oncol, 2013, 24 Suppl 6: vi144-vi148.
[7] Wirth A, Gospodarowicz M, Aleman BM, et al. Long-term outcome for gastric marginal zone lymphoma treated with radiotherapy: a retrospective, multi-centre, International Extranodal Lymphoma Study Group study[J]. Ann Oncol, 2013, 24(5): 1344-1351. doi: 10.1093/annonc/mds623
[8] Kalpadakis C, Pangalis GA, Angelopoulou MK, et al. Should rituximab replace splenectomy in the management of splenic marginal zone lymphoma?[J]. Best Pract Res Clin Haematol, 2018, 31(1): 65-72. doi: 10.1016/j.beha.2017.10.011
[9] Salar A, Domingo-Domenech E, Panizo C, et al. First-line response-adapted treatment with the combination of bendamustine and rituximab in patients with mucosa-associated lymphoid tissue lymphoma(MALT2008-01): a multicentre, single-arm, phase 2 trial[J]. Lancet Haematol, 2014, 1(3): e104-e111. doi: 10.1016/S2352-3026(14)00021-0
[10] Laribi K, Tempescul A, Ghnaya H, et al. The bendamustine plus rituximab regimen is active against primary nodal marginal zone B-cell lymphoma[J]. Hematol Oncol, 2017, 35(4): 536-541. doi: 10.1002/hon.2334
[11] CencinI E, Fabbri A, Schiattone L, et al. Efficacy and safety of rituximab plus bendamustine for gastric marginal zone lymphoma[J]. Leuk Lymphoma, 2019, 60(3): 833-835. doi: 10.1080/10428194.2018.1504938
[12] Castelli R, Bergamaschini L, Deliliers GL. First-line treatment with bendamustine and rituximab, in patients with intermediate-/high-risk splenic marginal zone lymphomas[J]. Med Oncol, 2017, 35(2): 15.
[13] Kang HJ, Kim WS, Kim SJ, et al. Phase Ⅱ trial of rituximab plus CVP combination chemotherapy for advanced stage marginal zone lymphoma as a first-line therapy: Consortium for Improving Survival of Lymphoma(CISL)study[J]. Ann Hematol, 2012, 91(4): 543-551. doi: 10.1007/s00277-011-1337-6
[14] Zucca E, Conconi A, Martinelli G, et al. Final Results of the IELSG-19 Randomized Trial of Mucosa-Associated Lymphoid Tissue Lymphoma: Improved Event-Free and Progression-Free Survival With Rituximab Plus Chlorambucil Versus Either Chlorambucil or Rituximab Monotherapy[J]. J Clin Oncol, 2017, 35(17): 1905-1912. doi: 10.1200/JCO.2016.70.6994
[15] Zucca E, Conconi A, Laszlo D, et al. Addition of rituximab to chlorambucil produces superior event-free survival in the treatment of patients with extranodal marginal-zone B-cell lymphoma: 5-year analysis of the IELSG-19 Randomized Study[J]. J Clin Oncol, 2013, 31(5): 565-572. doi: 10.1200/JCO.2011.40.6272
[16] ChesoN BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification[J]. J Clin Oncol, 2014, 32(27): 3059-3068. doi: 10.1200/JCO.2013.54.8800
[17] Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial[J]. Lancet, 2013, 381(9873): 1203-1210. doi: 10.1016/S0140-6736(12)61763-2
[18] Flinn IW, Van Der Jagt R, Kahl BS, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study[J]. Blood, 2014, 123(19): 2944-2952. doi: 10.1182/blood-2013-11-531327
[19] Flinn IW, Van Der Jagt R, Kahl B, et al. First-Line Treatment of Patients With Indolent Non-Hodgkin Lymphoma or Mantle-Cell Lymphoma With Bendamustine Plus Rituximab Versus R-CHOP or R-CVP: Results of the BRIGHT 5-Year Follow-Up Study[J]. J Clin Oncol, 2019, 37(12): 984-991. doi: 10.1200/JCO.18.00605
[20] Iannitto E, Bellei M, Amorim S, et al. Efficacy of bendamustine and rituximab in splenic marginal zone lymphoma: results from the phase Ⅱ BRISMA/IELSG36 study[J]. Br J Haematol, 2018, 183(5): 755-765. doi: 10.1111/bjh.15641
[21] Morigi A, Argnani L, Lolli G, et al. Bendamustine-rituximab regimen in untreated indolent marginal zone lymphoma: experience on 65 patients[J]. Hematol Oncol, 2020, 38(4): 487-492. doi: 10.1002/hon.2773
[22] Salar A, Domingo-Domenech E, Panizo C, et al. Long-term results of a phase 2 study of rituximab and bendamustine for mucosa-associated lymphoid tissue lymphoma[J]. Blood, 2017, 130(15): 1772-1774. doi: 10.1182/blood-2017-07-795302
[23] 郜桂菊, 张福杰, 姚均, 等. HIV感染者/AIDS患者CD4+细胞计数与机会性感染对应关系的临床分析[J]. 中国艾滋病性病, 2005, 11(4): 241-243. https://www.cnki.com.cn/Article/CJFDTOTAL-XBYA200504000.htm
[24] Alderuccio JP, Beaven AW, Shouse G, et al. Frontline Bendamustine and Rituximab in Extranodal Marginal Zone Lymphoma: An International Analysis[J]. Blood, 2020, 136(Supplement 1): 2-3.
-




 下载:
下载: