Value of plasma fibrinogen level before treatment in prognosis of patients with diffuse large B-cell lymphoma
-
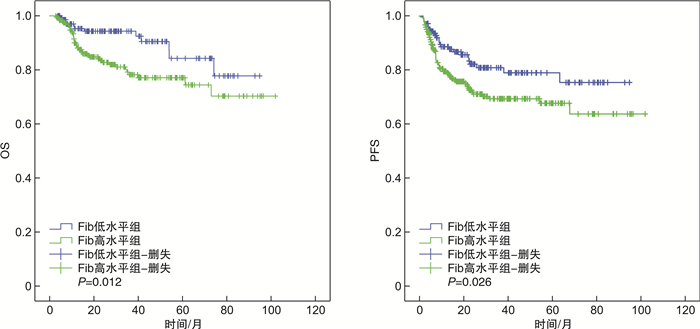
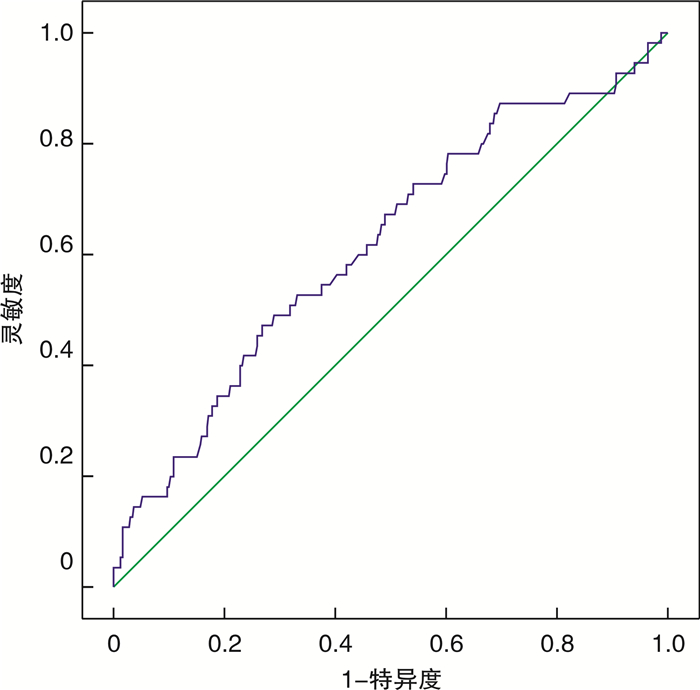
摘要: 目的 探讨治疗前血浆纤维蛋白原(Fib)水平在初治弥漫大B细胞淋巴瘤(DLBCL)患者预后判断中的价值。方法 回顾性分析符合入选标准的389例初治DLBCL患者治疗前血浆Fib水平与患者临床病理特征及预后的关系。采用受试者工作特征曲线确定治疗前血浆Fib截断值,按截断值将患者分为Fib高水平组(245例)和Fib低水平组(144例)。比较2组患者临床病理特征、总生存期(OS)及疾病无进展生存期(PFS)的差异,单因素和多因素回归分别分析初治DLBCL患者临床病理特征与预后的关系。结果 治疗前血浆Fib高水平组DLBCL患者中,血清乳酸脱氢酶(LDH)水平高于正常上限、Ann Arbor分期Ⅲ~Ⅳ、B症状、更高的国际预后指数(IPI)和NCCN-IPI评分的患者比例显著高于Fib低水平组。LDH水平高于正常、ECOG评分2~4分、Ann Arbor分期Ⅲ~Ⅳ、结外侵犯部位>1处、Fib高水平的初治DLBCL患者的OS、PFS较其他患者更短。多因素分析显示,LDH水平高于正常、ECOG评分2~4分是初治DLBCL患者OS的独立不良预后因子,LDH水平高于正常、结外侵犯部位>1处是初治DLBCL患者PFS的独立不良预后因子,治疗前血浆Fib高水平不是初治DLBCL患者OS、PFS的独立不良预后因子。结论 治疗前血浆Fib高水平与初治DLBCL患者不良预后相关,但并不是独立不良预后因子。Abstract: Objective To explore the prognostic value of plasma fibrinogen(Fib) level before treatment in patients with newly diagnosed diffuse large B-cell lymphoma(DLBCL).Methods The association of plasma Fib level before treatment and clinicopathological characteristics and prognosis of 389 patients with newly diagnosed DLBCL who met inclusion criteria was analyzed retrospectively. The cut-off value of plasma Fib level before treatment was determined by a receiver operator characteristic curve. Patients were divided into high level group(245 cases) and low level group(144 cases) according to the cut-off value. The differences in clinicopathological characteristics, overall survival(OS) and disease progression-free survival(PFS) between the two groups were compared. The relationship of clinicopathological characteristics and prognosis in patients with newly diagnosed DLBCL was analyzed by univariate and multivariate regression analysis.Results The proportion of patients with serum lactate dehydrogenase(LDH)>upper limit of normal, Ann Arbor stage Ⅲ-Ⅳ, B symptoms, higher international prognostic index(IPI) score and NCCN-IPI score was significantly higher in high plasma Fib group than those in low level group. Newly diagnosed DLBCL patients with LDH>upper limit of normal, or ECOG score 2-4, or Ann Arbor stage Ⅲ-Ⅳ, or more than 1 site of extranodal diseases, or high plasma Fib level had shorter OS and PFS than others. Multivariate analysis showed that LDH>upper limit of normal and ECOG score 2-4 were independent poor prognostic factors for OS of patients with newly diagnosed DLBCL. LDH>upper limit of normal and more than 1 site of extranodal diseases were independent poor prognostic factors for PFS of patients with newly diagnosed DLBCL. High plasma Fib level before treatment was not an independent poor prognostic factor for OS and PFS of patients with newly diagnosed DLBCL.Conclusion High plasma Fib level before treatment is associated with poor prognosis for patients with newly diagnosed DLBCL, but not an independent poor prognostic factor.
-
Key words:
- diffuse large B-cell lymphoma /
- fibrinogen /
- prognosis
-

-
表 1 治疗前血浆Fib水平与患者临床特征的关系
例 特征 Fib水平 P Fib低水平组
(144例)Fib高水平组
(245例)年龄 0.66 >60岁 48 88 ≤60岁 96 157 性别 1.00 男 87 147 女 57 98 LDH水平 < 0.01 >正常上限 52 139 正常 92 106 ECOG评分 0.26 0~1分 116 185 2~4分 28 60 Ann Arbor分期 0.04 Ⅰ~Ⅱ期 63 81 Ⅲ~Ⅳ期 81 164 结外病灶 0.27 >1 43 88 ≤1 101 157 B症状 0.02 无 117 172 有 27 73 IPI评分 0.02 低危(0~1分) 69 82 低中危(2分) 33 55 高中危(3分) 26 62 高危(4~5分) 16 46 NCCN-IPI评分 0.01 低危(0~1分) 38 41 低中危(2~3分) 67 101 高中危(4~5分) 26 79 高危(≥6分) 13 24 细胞亚型 0.49 生发中心 52 94 非生发中心 83 142 不能分类 9 9 表 2 影响DLBCL患者OS的单因素和多因素分析
特征 单因素分析 多因素分析 风险比(95%CI) P 风险比(95%CI) P 年龄(>60岁vs ≤60岁) 0.60(0.35~1.03) 0.06 性别(男vs女) 1.21(0.71~2.06) 0.49 B症状(有vs无) 2.60(1.53~4.43) < 0.01 LDH水平(>正常上限vs正常) 4.68(2.41~9.06) < 0.01 3.72(1.87~7.40) < 0.01 ECOG评分(2~4 vs 0~1) 3.26(1.92~5.55) < 0.01 2.20(1.26~3.82) < 0.01 Ann Arbor分期(Ⅲ~Ⅳ vs Ⅰ~Ⅱ) 2.83(1.43~5.63) < 0.01 结外病灶(>1 vs ≤1) 2.15(1.27~3.66) < 0.01 细胞亚型(生发中心vs非生发中心) 1.67(0.90~3.09) 0.11 Fib水平(高vs低) 2.22(1.17~4.21) 0.02 表 3 影响DLBCL患者PFS的单因素和多因素分析
特征 单因素分析 多因素分析 风险比(95%CI) P 风险比(95%CI) P 年龄(>60岁vs ≤60岁) 1.37(0.89~2.10) 0.16 性别(男vs女) 1.08(0.71~1.65) 0.71 B症状(有vs无) 1.51(0.97~2.36) 0.07 LDH水平(>正常上限vs正常) 2.52(1.61~3.92) < 0.01 2.17(1.37~3.44) < 0.01 ECOG评分(2~4 vs 0~1) 2.24(1.45~3.45) < 0.01 Ann Arbor分期(Ⅲ~Ⅳ vs Ⅰ~Ⅱ) 2.49(1.49~4.19) < 0.01 结外病灶(>1 vs ≤1) 2.14(1.41~3.25) < 0.01 1.73(1.12~2.66) 0.01 细胞亚型(生发中心vs非生发中心) 1.50(0.95~2.39) 0.09 Fib水平(高vs低) 1.69(1.06~2.70) 0.03 -
[1] Li S, Young KH, Medeiros LJ. Diffuse large B-cell lymphoma[J]. Pathology, 2018, 50(1): 74-87. doi: 10.1016/j.pathol.2017.09.006
[2] Sehn LH, Gascoyne RD. Diffuse large B-cell lymphoma: optimizing outcome in the context of clinical and biologic heterogeneity[J]. Blood, 2015, 125(1): 22-32. doi: 10.1182/blood-2014-05-577189
[3] Barrans SL, Crouch S, Care MA, et al. Whole genome expression profiling based on paraffin embedded tissue can be used to classify diffuse large B-cell lymphoma and predict clinical outcome[J]. Br J Haematol, 2012, 159(4): 441-453. doi: 10.1111/bjh.12045
[4] Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study[J]. Blood, 2017, 130(16): 1800-1808. doi: 10.1182/blood-2017-03-769620
[5] Salles G, Barrett M, Foà R, et al. Rituximab in B-cell hematologic malignancies: a review of 20 years of clinical experience[J]. Adv Ther, 2017, 34(10): 2232-2273. doi: 10.1007/s12325-017-0612-x
[6] Zhang K, Xu Y, Tan S, et al. The association between plasma fibrinogen levels and lung cancer: a meta-analysis[J]. J Thorac Dis, 2019, 11(11): 4492-4500. doi: 10.21037/jtd.2019.11.13
[7] Lin Y, Liu Z, Qiu Y, et al. Clinical significance of plasma D-dimer and fibrinogen in digestive cancer: a systematic review and meta-analysis[J]. Eur J Surg Oncol, 2018, 44(10): 1494-1503. doi: 10.1016/j.ejso.2018.07.052
[8] Sun Y, Zhang Y, Huang Z, et al. Combination of preoperative plasma fibrinogen and neutrophil-to-nymphocyte ratio(the F-NLR score)as a prognostic marker of locally advanced rectal vancer following preoperative chemoradiotherapy[J]. World J Surg, 2020, 44(6): 1975-1984. doi: 10.1007/s00268-020-05407-3
[9] Wang Z, Fan H, Wang W, et al. High preoperative plasma fibrinogen independently predicts a poor prognosis in patients with nonmetastatic RCC[J]. J Cancer, 2020, 11(9): 2401-2407. doi: 10.7150/jca.40961
[10] Li X, Shu K, Zhou J, et al. Preoperative plasma fibrinogen and D-dimer as prognostic biomarkers for non-muscle-invasive bladder cancer[J]. Clin Genitourin Cancer, 2020, 18(1): 11-19.e1. doi: 10.1016/j.clgc.2019.10.025
[11] Wu M, Pan Y, Jia Z, et al. Preoperative plasma fibrinogen and serum albumin score is an independent prognostic factor for resectable stage Ⅱ-Ⅲ gastric cancer[J]. Dis Markers, 2019, 2019: 9060845.
[12] Troppan KT, Melchardt T, Wenzl K, et al. The clinical significance of fibrinogen plasma levels in patients with diffuse large B cell lymphoma[J]. J Clin Pathol, 2016, 69(4): 326-330. doi: 10.1136/jclinpath-2015-203356
[13] Niu JY, Tian T, Zhu HY, et al. Hyperfibrinogenemia is a poor prognostic factor in diffuse large B cell lymphoma[J]. Ann Hematol, 2018, 97(10): 1841-1849. doi: 10.1007/s00277-018-3382-x
[14] Shehata AMF, Aldesoky AI, Gohar SF. Plasma fibrinogen level as possible prognostic biomarker in diffuse large B-cell lymphoma[J]. Hematology, 2019, 24(1): 103-107. doi: 10.1080/10245332.2018.1519932
[15] Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma[J]. J Clin Oncol, 2007, 25(5): 579-586. doi: 10.1200/JCO.2006.09.2403
[16] Ayers EC, Li S, Medeiros LJ, et al. Outcomes in patients with aggressive B-cell non-Hodgkin lymphoma after intensive frontline treatment failure[J]. Cancer, 2020, 126(2): 293-303. doi: 10.1002/cncr.32526
[17] 徐卫, 梁金花. 弥漫大B细胞淋巴瘤新基因分型及分子靶向的治疗进展[J]. 临床血液学杂志, 2020, 33(9): 594-598. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXZ202009002.htm
[18] 邵奕, 唐善浩, 陆滢, 等. R-CHOP方案治疗大包块和(或)结外多部位累及的弥漫大B细胞淋巴瘤患者2年疗效和安全性观察[J]. 临床血液学杂志, 2020, 33(7): 481-492. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXZ202007010.htm
[19] Abdol Razak NB, Jones G, Bhandari M, et al. Cancer-associated thrombosis: an overview of mechanisms, risk factors, and treatment[J]. Cancers(Basel), 2018, 10(10): 380.
[20] Sahni A, Simpson-Haidaris PJ, Sahni SK, et al. Fibrinogen synthesized by cancer cells augments the proliferative effect of fibroblast growth factor-2(FGF-2)[J]. J Thromb Haemost, 2008, 6(1): 176-183.
-




 下载:
下载: