Guiding principle for the administration of amphotericin B colloidal dispersion for injection
-
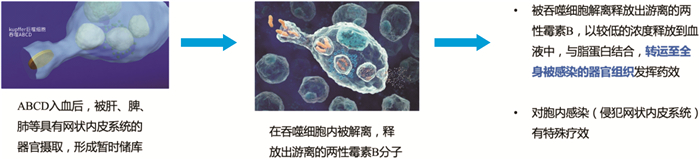
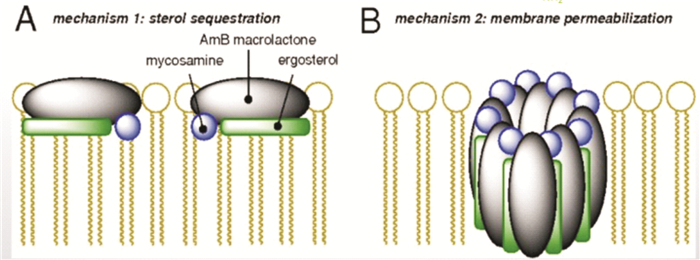
摘要: 两性霉素B胆固醇硫酸酯复合物是两性霉素B的含脂制剂,经过结构修饰后显著降低传统两性霉素B的肾毒性,提升临床有效用药剂量,实现更好的治疗效果。抗真菌药物的规范化用药至关重要,本指导原则旨在为两性霉素B胆固醇硫酸酯复合物在临床的安全和有效使用提供建议。
-
关键词:
- 两性霉素B胆固醇硫酸酯复合物 /
- 指导原则
Abstract: Amphotericin B colloidal dispersion(ABCD) is a novel formulation of amphotericin B with a nearly 1∶1 molar ratio of cholesteryl sulfate to amphotericin B that forms uniform disk-shaped particles 115 nm in diameter and 4 nm thick. Due to the structural modification, ABCD achieved higher drug doses and better therapy efficacy with reduced renal toxicity. Standardized medication for antifungal drugs is very important, therefore the guiding principle aims to provide effective and practical instructions for the use of ABCD.-
Key words:
- amphotericin B colloidal dispersion /
- guiding principle
-

-
表 1 权威指南对ABCD抗真菌治疗的推荐
指南和规范 内容 推荐级别 2015年ECIL-6指南:白血病和造血干细胞移植患者侵袭性念珠菌病、曲霉菌病和毛霉菌病的治疗 推荐ABCD用于念珠菌血症初始一线治疗和目标治疗的一线治疗:白念珠菌、光滑念珠菌、克鲁斯念珠菌 BⅡ级 推荐ABCD用于侵袭性曲霉菌病的一线治疗 CⅠ级 推荐ABCD用于毛霉菌病的一线治疗 CⅡ级 2016年美国感染病学会曲霉病诊断处理实践指南 采用ABCD治疗侵袭性曲霉菌病时,推荐剂量为:3~6 mg/(kg·d) 当无法应用伏立康唑时,两性霉素B及其含脂制剂是曲霉病初始治疗及补救治疗的选择 强推荐 肺外曲霉病:不能耐受伏立康唑或用后无效的中枢神经系统曲霉病患者,可采用两性霉素B含脂制剂;推荐手术治疗联合全身药物治疗(可采用伏立康唑或两性霉素B含脂制剂)治疗侵袭性曲霉性鼻窦炎;对于曲霉心内膜炎患者,推荐早期手术干预并联合抗真菌治疗,初始治疗推荐伏立康唑或两性霉素B含脂制剂;对于肝曲霉病患者,建议采用伏立康唑或两性霉素B含脂制剂作为初始治疗 强推荐 对于长期中性粒细胞减少的侵袭性曲霉病高危患者,经广谱抗菌药物治疗仍发热,推荐进行经验性抗曲霉治疗,可选用的抗真菌药物有两性霉素B含脂制剂 强推荐 2017年曲霉菌病的诊断和管理:ESCMID-ECMM-ERS指南执行摘要 异基因造血干细胞移植(伴或不伴中性粒细胞减少)或其他非中性粒细胞减少的肺曲霉菌病患者,推荐使用ABCD 4~6 mg/kg一线治疗 Ⅰ类证据,D级推荐 血液恶性肿瘤或造血干细胞移植的化疗、中性粒细胞减少 < 500/μL≥96 h、发热(>38℃)、肠外广谱抗菌治疗≥96 h(有些中心认为48 h)的患者,推荐使用ABCD 4 mg/kg经验性治疗 Ⅰ类证据,C级推荐 推荐难治性肺曲霉菌病的血液病患者使用ABCD治疗 2019年ECMM全球指南-毛霉菌病的诊断和管理 推荐ABCD用于毛霉菌的抢救治疗(难治性或者一线治疗失败或不耐受的患者) 对于初次使用艾沙康唑或泊沙康唑治疗失败的毛霉菌患者,推荐采用ABCD治疗 强到中等强度推荐 表 2 ABCD的稀释建议
注射用两性霉素B胆固醇硫酸酯复合物剂量 重溶体积(吸取药液体积) 5%葡萄糖注射液输注袋体积(输液总体积) 10~35 mg 2~7 mL 250 mL 35~70 mg 7~14 mL 250~500 mL 70~175 mg 14~35 mL 500 mL 175~300 mg 35~70 mL 700 mL 350~1000 mg 70~200 mL 1000 mL -
[1] Bowden RA, Cays M, Gooley T, et al. Phase Ⅰ study of amphotericin B colloidal dispersion for the treatment of invasive fungal infections after marrow transplant[J]. J Infect Dis, 1996, 173(5): 1208-1215. doi: 10.1093/infdis/173.5.1208
[2] Gurwith M. Clinical efficacy of amphotericin B colloidal dispersion against infections caused by Aspergillus spp[J]. Chemotherapy, 1999, 45 Suppl 1: 34-38.
[3] Dupont B. Clinical efficacy of amphotericin B colloidal dispersion against infections caused by Candida spp[J]. Chemotherapy, 1999, 45 Suppl 1: 27-33.
[4] White MH, Bowden RA, Sandler ES, et al. Randomized, double-blind clinical trial of amphotericin B colloidal dispersion vs. amphotericin B in the empirical treatment of fever and neutropenia[J]. Clin Infect Dis, 1998, 27(2): 296-302. doi: 10.1086/514672
[5] White MH, Anaissie EJ, Kusne S, et al. Amphotericin B colloidal dispersion vs. amphotericin B as therapy for invasive aspergillosis[J]. Clin Infect Dis, 1997, 24(4): 635-642.
[6] Anaissie EJ, Mattiuzzi GN, Miller CB, et al. Treatment of invasive fungal infections in renally impaired patients with amphotericin B colloidal dispersion[J]. Antimicrob Agents Chemother, 1998, 42(3): 606-611. doi: 10.1128/AAC.42.3.606
[7] Herbrecht R, Letscher-Bru V, Bowden RA, et al. Treatment of 21 cases of invasive mucormycosis with amphotericin B colloidal dispersion[J]. Eur J Clin Microbiol Infect Dis, 2001, 20(7): 460-466.
[8] Bowden R, Chandrasekar P, White MH, et al. A double-blind, randomized, controlled trial of amphotericin B colloidal dispersion versus amphotericin B for treatment of invasive aspergillosis in immunocompromised patients[J]. Clin Infect Dis, 2002, 35(4): 359-366. doi: 10.1086/341401
[9] Tissot F, Agrawal S, Pagano L, et al. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients[J]. Haematologica, 2017, 102(3): 433-444. doi: 10.3324/haematol.2016.152900
[10] Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America[J]. Clin Infect Dis, 2016, 63(4): e1-e60. doi: 10.1093/cid/ciw326
[11] Ullmann AJ, Aguado JM, Arikan-Akdagli S, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline[J]. Clin Microbiol Infect, 2018, 24 Suppl 1: e1-e38.
[12] Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium[J]. Lancet Infect Dis, 2019, 19(12): e405-e421. doi: 10.1016/S1473-3099(19)30312-3
[13] Meletiadis J, Chanock S, Walsh TJ. Defining targets for investigating the pharmacogenomics of adverse drug reactions to antifungal agents[J]. Pharmacogenomics, 2008, 9(5): 561-584. doi: 10.2217/14622416.9.5.561
[14] Herbrecht R, Letscher V, Andres E, et al. Safety and efficacy of amphotericin B colloidal dispersion. An overview[J]. Chemotherapy, 1999, 45 Suppl 1: 67-76.
[15] David L, Kristin D, Mirando M, et al. Pre-medication practices and incidence of infusion-related reactions in patients receiving AMPHOTECw: data from the Patient Registry of Amphotericin B Cholesteryl Sulfate Complex for Injection Clinical Tolerability(PRoACT)registry[J]. J Antimicrob Chemother, 2008, 62(6): 1392-1400. doi: 10.1093/jac/dkn394
[16] Baley JE, Meyers C, Kliegman RM. Pharmacokinetic outcome and toxic effects of amphotericin B and 5 fluorocyto-sine in neonates[J]. J Pediatr, 1990, 116(5): 791-797. doi: 10.1016/S0022-3476(05)82674-5
[17] Hoitsma JA, Wetzels JFM, Koene R. Drug-induced nephrotoxicity. Aetiology, clinical features and management[J]. Drug Safety, 1991, 6(2): 131-147. doi: 10.2165/00002018-199106020-00004
[18] Butler KM, Rench MA, Baker CJ. Amphotericin B as a single agent in the treatment of systemic candidiasis in neonates[J]. Pediatr Infect Dis J, 1990, 9(1): 51-56. doi: 10.1097/00006454-199001000-00012
[19] Fanos V, Cataldi L. Amphotericin B-induced nephrotoxicity: a review[J]. J Chemother, 2000, 12(6): 463-670. doi: 10.1179/joc.2000.12.6.463
[20] 吴文芳, 杜兰云, 董结兰. 低钾血症的病因和治疗进展[J]. 临床合理用药杂志, 2018, 11(1): 174-175. https://www.cnki.com.cn/Article/CJFDTOTAL-PLHY201801101.htm
[21] 殷长春. 低钾血症患者的急救护理体会[J]. 临床医药文献电子杂志, 2020, 7(23): 102-102. https://www.cnki.com.cn/Article/CJFDTOTAL-LCWX202023098.htm
-




 下载:
下载: