Efficacy and safety of ruxolitinib in the treatment of steroid-refractory graft-versus-host disease: a meta-analysis
-
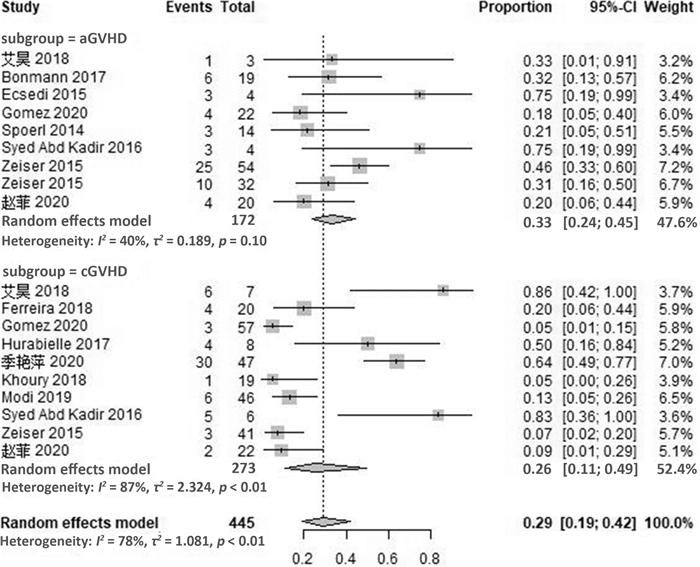
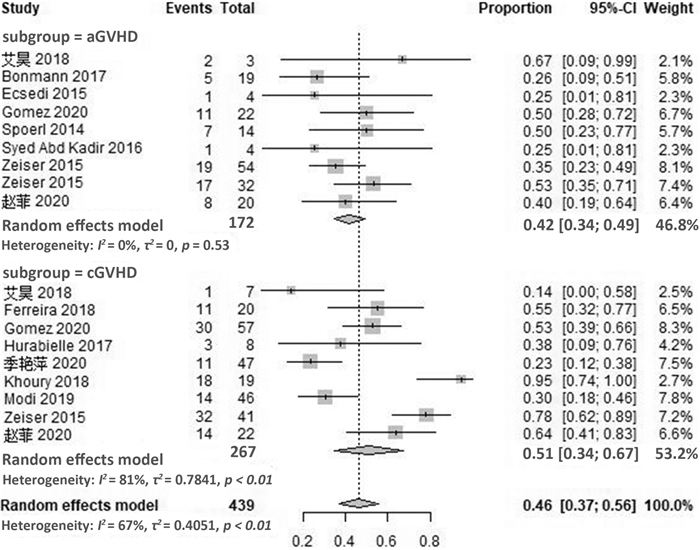
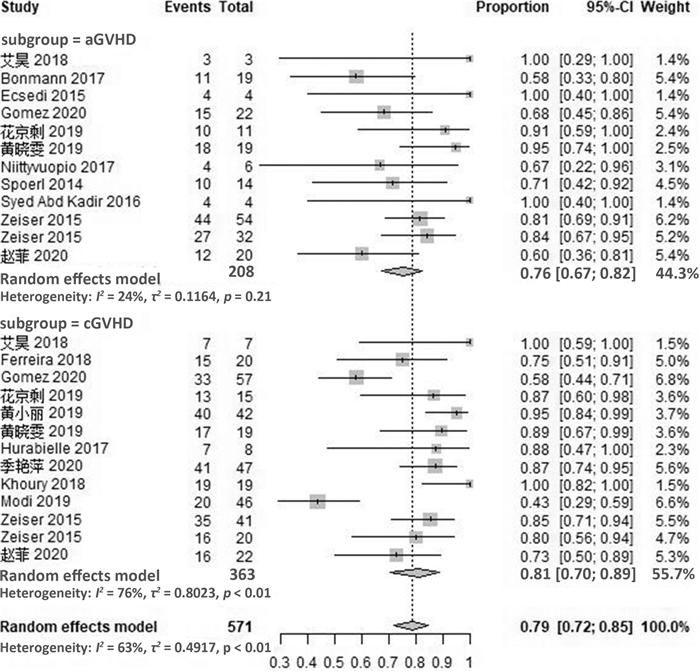
摘要: 目的 系统评价芦可替尼治疗激素难治性移植物抗宿主病的疗效和安全性。方法 计算机检索PubMed、Embase、Cochrane图书馆及ClinicalTrials.gov英文数据库、中国期刊全文数据库(CNKI)、维普和万方数据库,检索截至日期2020年8月。根据研究特点对纳入研究进行质量评价后采用R软件进行统计分析。结果 最终纳入21篇文献,共609例患者。Meta分析结果显示,芦可替尼治疗激素难治性移植物抗宿主病患者的有效率(ORR)为79%(95%CI72%~85%),完全缓解率(CR)为29%(95%CI19%~42%),部分缓解率(PR)为46%(95%CI37%~56%)。其中,急性移植物抗宿主病的ORR为76%(95%CI67%~82%),CR率为33%(95%CI24%~45%),PR率为42%(95%CI34%~49%);慢性移植物抗宿主病的ORR为81%(95%CI70%~89%),CR率为26%(95%CI11%~49%),PR率为51%(95%CI34%~67%)。在安全性方面,使用芦可替尼后骨髓抑制、巨细胞病毒再激活及其他感染的不良反应率增加,血细胞减少发生率为17%(95%CI10%~28%),巨细胞病毒再激活率为21%(95%CI15%~29%),其他感染发生率为18%(95%CI9%~33%)。结论 芦可替尼用于成人急性GVHD和慢性GVHD患者的挽救性治疗,可获得较高的有效率,需关注骨髓抑制和感染的发生。
-
关键词:
- 芦可替尼 /
- 异基因造血干细胞移植 /
- 移植物抗宿主病 /
- 挽救性治疗 /
- meta分析
Abstract: Objective To systematically evaluate the efficacy and safety of ruxolitinib in patients with steroid-refractory graft-versus-host disease(SR-GVHD).Methods The database including PubMed, Embase, Cochrane Library, ClinicalTrials. gov in English, CNKI, CBM, VIP and Wanfang were searched, and the references of the included studies were retrieved manually, with the retrieval time from inception to August 2020. The quality evaluation of included criteria based on the characteristics of the literature was evaluated, and the meta-analysis was conducted by using R version3.5 software.Results A total of 21 studies involving 609 patients were included. The results of meta-analysis showed that the overall response rate(ORR), complete remission(CR) rate and partial remission(PR) rate of ruxolitinib in the treatment of SR-GVHD were 79%(95%CI72%-85%), 29%(95%CI19%-42%), 46%(95%CI37%-56%), respectively. For patients with SR-aGVHD, the ORR, CR rate and PR rate were 76%(95%CI67%-82%), 33%(95%CI24%-45%) and 42%(95%CI34%-49%), while for patients with SR-cGVHD, the ORR, CR rate and PR rate were 81%(95%CI70%-89%), 26%(95%CI11%-49%) and 51%(95%CI34%-67%). In terms of safety, the adverse events of myelosuppression, cytomegalovirus reactivation and other infections increased after the use of ruxolitinib. The incidence of hemocytopenia was 17%(95%CI10%-28%), and the reactivation rate of cytomegalovirus was 21%(95%CI15%-29%), while the incidence of other infections was 18%(95%CI9%-33%).Conclusion Ruxolitinib can obtain high effective rate in the salvage treatment of adult patients with aGVHD and cGVHD. Attention should be paid to the occurrence of bone marrow suppression and infection. -

-
表 1 纳入文献基本特征及文献质量评估
纳入研究 例数 中位年龄/岁 男∶女/例 原发疾病/例 移植类型/例 GVHD分型(急性∶慢性∶混合)/例 随访时间/月 NOS评分 Assouan(2018)[4] 10 52 (26~65) 7∶3 ALL(4),MDS(2),MF(2),HL(1),MPN(1) MSD(5),MUD(5) 8∶0∶2 4.5 (1.4~15.2) 7 Bonmann(2017)[7] 19 58 (18~74) 12∶7 NA MSD(3),MUD(12),MMUD(3) 19∶0∶2 NA 6 Ecsedi(2015)[8] 5 43 (21~66) 5∶0 AML(1),ALL(1),PM(2),T-PLL(1) MUD(2),MMUD(1),Sibling(2) 4∶2∶1 3.0 (2.3~9.3) 3 Ferreira(2018)[9] 20 46.5 (23~68) 11∶9 NA MRD(10),URD(4),Haploidentical(6) 0∶20∶2 12.0 (4.0~19.0) 7 Escamilla Gomez(2020)[10] 79 51 (0~73) 48∶31 AML(30),ALL(12),MDS(11),MF(4),HL(2),NHL(13),MM(3),其他(4) MSD(33),URD(39),Haploidentical(7) 22∶57∶2 NA 7 Hurabielle(2017)[11] 12 47 (21~67) 7∶5 AML(4),ALL(2),MDS(2),MF(2),FL(1),DLBCL(1) MUD(8),Sibling(4) 0∶12∶2 >6 5 Khoury(2018)[6] 19 53 (28~73) 11∶8 ALL(2),CLL(1),CMML(3),MDS(5),HL(1),MPN(1),T细胞淋巴瘤(1) URD(13),Sibling(6) 0∶19∶0 最大随访时间:24.0 5 Lupo-Stanghellini(2017)[12] 5 57(39~67) NA NA NA 0∶4∶1 28.9 7 Modi(2019)[5] 46 49 (21~77) 26∶20 AML(15),ALL(10),CLL(2),MDS(6),MF(2),HL(2),NHL(6),急性红系白血病(1) MUD(16),MMUD(5),Sibling(10) 0∶46∶0 最大随访时间:8.7 7 Niittyvuopio(2017)[13] 6 50 (19~59) 6∶0 AML(3),ALL(1),MDS(1),MM(1) MUD(4),Sibling(2) 6∶0∶0 NA 4 Poyatos-Ruiz(2016)[14] 13 46 (32~51) 6∶7 NA NA 5∶8∶0 NA 5 Spoerl(2014)[15] 14 NA NA NA NA 10∶4∶0 NA 4 Syed Abd Kadir(2016)[16] 9 63 (46~74) NA NA MSD(2),MUD(3),MMUD(4) NA NA 4 Zeiser(2015)[17] 95 aGVHD:51 (21~75);cGVHD:55 (22~74) 66∶29 aGVHD:AML(26),ALL(6),CLL(2),MDS(5),MF(2),HL(4),MM(4),T-PLL(1),CMML(1),HLH(1);cGVHD:AML(21),CLL(5),MDS(3),MF(5),HL(3),MPN(1),MM(1),CMML(1) NA 54∶41∶0 aGVHD:6.2 (0.7~25.0);cGVHD:5.2 (0.7~31.5) 6 Zeiser(2015)[18] 52 NA NA NA NA 32∶20∶0 NA 6 赵菲(2020)[21] 42 40(8~63) 29∶13 aGVHD:AML(9),ALL(1),MDS(4),NHL(1),AA(2),CML(2),CMML(1);cGVHD:AML(7),ALL(5),MDS(4),NHL(1),AA(3),CML(1),MPN(1) aGVHD:MSD(5),MMRD(13),URD(2);cGVHD:MSD(16),MMRD(4),URD(2) 20∶22∶0 8.8 (0.2~25.0) 5 黄小丽(2019)[24] 42 23(3~55) 19∶23 AML(14),ALL(9),MDS(5),AA(6),HLH(1),CML(2),HAL(2),地贫(3) MSD(13),MMRD(29) 0∶42∶0 4.0 (1.0~19.0) 6 季艳萍(2020)[23] 47 25 (7~54) 23∶24 AML(30),ALL(9),MDS(4),SAA(2),CML(2) NA 0∶47∶0 6.9 (1.1~13.7) 6 花京剩(2019)[20] 26 22 (10~51) 13∶13 AML(14),ALL(8),HAL(2),NHL(1),MDS(1) NA 11∶15∶0 3.0 (2.0~10.0) 4 艾昊(2018)[19] 10 30(17~37) 7∶3 AML(6),ALL(2),CMML(1),MDS(1) NA 3∶7∶0 3.3(2.0~5.0) 4 黄晓雯(2019)[22] 38 26(10~58) 19∶19 AML(15),ALL(17),HAL(2),NHL(2),MDS(2) NA 19∶19∶0 8.0 (1.0~15.0) 5 ALL:急性淋巴细胞白血病;AML:急性髓系白血病;MDS骨髓增生异常综合征;HAL:急性杂合细胞白血病;CMML:慢性粒单核细胞白血病;MF:骨髓纤维化;HL:霍奇金淋巴瘤;DLBCL:弥漫大B细胞淋巴瘤;FL:滤泡淋巴瘤;MPN:骨髓增殖性肿瘤;T-PLL:T淋巴细胞白血病;CLL:慢性淋巴细胞白血病;NHL:非霍奇金淋巴瘤;MM:多发性骨髓瘤;HLH:噬血细胞性淋巴组织细胞增多症;AA:再生障碍性贫血;SAA:重型再生障碍性贫血;MMRD:亲缘半相合供者;MMUD:非亲缘半相合供者;MSD:匹配同胞供者;URD:非血缘(无关)供者;MUD:相合无关(非血缘)供者;Haploidentical:单倍体;Sibling:同胞供者;NA:不详。 -
[1] Penack O, Marchetti M, Ruutu T, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation[J]. Lancet Haematol, 2020, 7(2): e157-e167. doi: 10.1016/S2352-3026(19)30256-X
[2] Jaglowski M, Blazar BR. How ibrutinib, a B-cell malignancy drug, became an FDA-approved second-line therapy for steroid-resistant chronic GVHD[J]. Blood Adv, 2018, 2(15): 2012-2019. doi: 10.1182/bloodadvances.2018013060
[3] Schoemans HM, Lee SJ, Ferrara JL, et al. EBMT-NIH-CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment[J]. Bone Marrow Transplant, 2018, 53(11): 1401-1415. doi: 10.1038/s41409-018-0204-7
[4] Assouan D, Lebon D, Charbonnier A, et al. Ruxolitinib as a promising treatment for corticosteroid-refractory graft-versus-host disease[J]. Br J Haematol, 2018, 181(5): 687-689. doi: 10.1111/bjh.14679
[5] Modi B, Hernandez-Henderson M, Yang D, et al. Ruxolitinib as Salvage Therapy for Chronic Graft-versus-Host Disease[J]. Biol Blood Marrow Transplant, 2019, 25(2): 265-269. doi: 10.1016/j.bbmt.2018.09.003
[6] Khoury HJ, Langston AA, Kota VK, et al. Ruxolitinib: A steroid sparing agent in chronic graft-versus-host disease[J]. Bone Marrow Transplant, 2018, 53(7): 826-831. doi: 10.1038/s41409-017-0081-5
[7] Bonmann S, Christopeit M, Wolschke C, et al. Ruxolitinib plus Extracorporeal Photopheresis(ECP)may increase response in steroid refractory acute graft-versus-host disease(aGVHD)[J]. Bone Marrow Transplant, 2017, 52: 304-305. doi: 10.1038/bmt.2016.262
[8] Ecsedi M, Gerull S, Medinger M, et al. Efficacy of ruxolitinib in the treatment of steroid-refractory graft-versus-host disease[J]. Praxis, 2015, 104: 163-164.
[9] Ferreira AM, Pontes da Silva CA, Pereira AD, et al. Ruxolitinib in steroid-refractory chronic graft-versus-host disease: experience of a single center[J]. Bone Marrow Transplant, 2018, 53(4): 503-506. doi: 10.1038/s41409-017-0068-2
[10] Escamilla Gómez V, García-Gutiérrez V, López Corral L, et al. Ruxolitinib in refractory acute and chronic graft-versus-host disease: a multicenter survey study[J]. Bone Marrow Transplant, 2020, 55(3): 641-648. doi: 10.1038/s41409-019-0731-x
[11] Hurabielle C, Sicre de Fontbrune F, Moins-Teisserenc H, et al. Efficacy and tolerance of ruxolitinib in refractory sclerodermatous chronic graft-versus-host disease[J]. Br J Dermatol, 2017, 177(5): e206-e208.
[12] Lupo-Stanghellini MT, Corti C, Guggiari E, et al. Promising efficacy and safety profile of ruxolitinib in highly pre-treated chronic GvHD patients[J]. Bone Marrow Transplant, 2017, 52: 301. doi: 10.1038/bmt.2016.261
[13] Niittyvuopio R, Heiskanen J, Lindstrom V, et al. Ruxolitinib treatment for corticosteroid-resistant acute intestinal GVHD[J]. Bone Marrow Transplant, 2017, 52: 306.
[14] Poyatos-Ruiz LL, Vega-Coca MD, Flores-Moreno S, et al. Off-label use of ruxolitinib in refractory graft-versus-host disease after allogenic stem cell transplantation[J]. Value Health, 2016, 19(7): A576.
[15] Spoerl S, Maas-Bauer K, Verbeek M, et al. Response to JAK 1/2 inhibition in patients with corticosteroid-refractory acute graft-versus-host disease[J]. Blood, 2014, 124(21): 3934. doi: 10.1182/blood.V124.21.3934.3934
[16] Syed Abd Kadir S, Wolschke C, Ayuk F, et al. JAK-inhibition to treat steroid-refractory acute and chronic graft versus host disease(SR-GVHD)-a single center experience[J]. Bone Marrow Transplant, 2016, 51: S381.
[17] Zeiser R, Burchert A, Lengerke C, et al. Treatment of corticosteroid-refractory graft-versus-host disease with ruxolitinib in 95 patients[J]. Blood, 2015, 126(23): 858. doi: 10.1182/blood.V126.23.858.858
[18] Zeiser R, Lengerke C, Spoerl S, et al. High response rates in patients treated with ruxolitinib for corticosteroid-refractory graft-versus-host disease: Analysis including 13 transplant centers[J]. Bone Marrow Transplant, 2015, 50: S22.
[19] 艾昊, 符粤文, 王勇奇, 等. 芦可替尼挽救性治疗难治性急慢性移植物抗宿主病的疗效及安全性观察[J]. 中华血液学杂志, 2018, 39(12): 1026-1029. doi: 10.3760/cma.j.issn.0253-2727.2018.12.011
[20] 花京剩, 黄晓雯, 仇惠英. 低剂量芦可替尼在26例移植后急慢性移植物抗宿主病中的临床疗效和不良反应观察[J]. 临床血液学杂志, 2019, 32(11): 855-859. doi: 10.13201/j.issn.1004-2806.2019.11.008
[21] 赵菲, 王佳丽, 施圆圆, 等. 芦可替尼挽救性治疗激素难治性移植物抗宿主病: 一项单中心回顾性分析[J]. 临床血液学杂志, 2020, 33(1): 18-24. doi: 10.13201/j.issn.1004-2806.2020.01.005
[22] 黄晓雯, 鲍协炳, 仇惠英, 等. 小剂量芦可替尼治疗38例急慢性移植物抗宿主病的疗效观察[J]. 中华器官移植杂志, 2019, 40(12): 749-752. doi: 10.3760/cma.j.issn.0254-1785.2019.12.010
[23] 季艳萍, 汤宝林, 朱小玉, 等. 芦可替尼挽救性治疗慢性移植物抗宿主病的效果及安全性[J]. 中华医学杂志, 2020, 100(16): 1235-1239. doi: 10.3760/cma.j.cn112137-20190829-01917
[24] 黄小丽, 刘林, 袁忠涛, 等. 芦可替尼对慢性移植物抗宿主病患者的疗效及安全性研究[J]. 第三军医大学学报, 2019, 41(23): 2290-2295. https://www.cnki.com.cn/Article/CJFDTOTAL-DSDX201923009.htm
[25] Hattori K, Doki N, Kurosawa S, et al. Mycophenolate mofetil is effective only for involved skin in the treatment for steroid-refractory acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation[J]. Ann Hematol, 2017, 96(2): 319-321. doi: 10.1007/s00277-016-2854-0
[26] Yalniz FF, Hefazi M, McCullough K, et al. Safety and efficacy of infliximab therapy in the setting of steroid-refractory acute graft-versus-host disease[J]. Biol Blood Marrow Transplant, 2017, 23: 1478-1484. doi: 10.1016/j.bbmt.2017.05.001
[27] De Jong CN, Saes L, Klerk CPW, et al. Etanercept for steroid-refractory acute graft-versus-host disease: a single center experience[J]. PLos One, 2017, 12: e0187184. doi: 10.1371/journal.pone.0187184
[28] Spoerl S, Mathew NR, Bscheider M, et al. Activity oftherapeutic JAK 1/2 blockade in graft-versus-host disease[J]. Blood, 2014, 123: 3832-3842. doi: 10.1182/blood-2013-12-543736
[29] von Bubnoff N, Ihorst G, Grishina O, et al. Ruxolitinib in GvHD(RIG)study: A multicenter, randomized phase 2 trial to determine the response rate of Ruxolitinib and best available treatment(BAT)versus BAT in steroid-refractory acute graft-versus-host disease(aGvHD)(NCT02396628)[J]. BMC Cancer, 2018, 18(1): 1132. doi: 10.1186/s12885-018-5045-7
[30] Abedin S, McKenna E, Chhabra S, et al. Toxicity, and Infectious Complications in Ruxolitinib-Treated Patients with Corticosteroid-Refractory Graft-versus-Host Disease after Hematopoietic Cell Transplantation[J]. Biol Blood Marrow Transplant, 2019, 25(8): 1689-1694. doi: 10.1016/j.bbmt.2019.04.003
[31] Hui L, Qi L, Guoyu H, et al. Ruxolitinib for treatment of steroid-refractory graft-versus-host disease in adults: a systematic review and meta-analysis[J]. Expert Rev Hematol, 2020, 13(5): 565-575. doi: 10.1080/17474086.2020.1738214
[32] Przepiorka D, Luo L, Subramaniam S, et al. FDA approval summary: ruxolitinib for treatment of steroid-refractory acute graft-versus-host disease[J]. Oncologist, 2020, 25(2): e328-e334. doi: 10.1634/theoncologist.2019-0627
-




 下载:
下载: