Efficacy and safety of frontline FCR(fludarabine, cyclophosphamide, rituximab) with dose reduction of rituximab in chronic lymphocytic leukemia
-
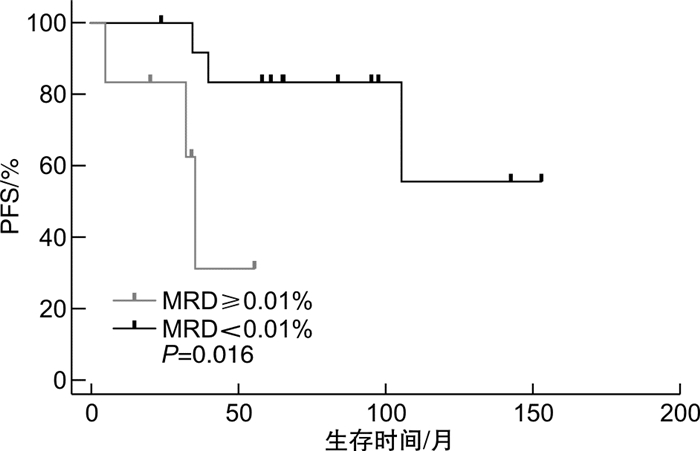
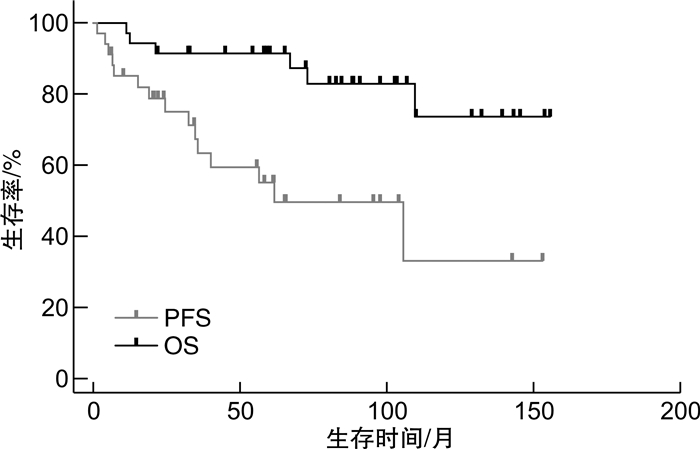
摘要: 目的 分析利妥昔单抗减量(375 mg/m2固定剂量)的氟达拉滨+环磷酰胺+利妥昔单抗(FCR)方案一线治疗慢性淋巴细胞白血病(CLL)的疗效,并探讨影响疗效的预后因素。方法 回顾性分析2009年9月—2021年6月在我院接受一线利妥昔单抗减量FCR方案治疗的35例CLL患者的临床资料。结果 35例患者中男25例,女10例,中位年龄58(42~75)岁,中位疗程数为6(2~6)个,总反应率为91.4%(32/35),15例(42.9%)达完全缓解,3例(8.6%)达骨髓未恢复的完全缓解,14例(40.0%)达部分缓解,2例(5.7%)疾病稳定,1例(2.9%)疾病进展。20例患者进行了骨髓微小残留病(MRD)检测,14例(70.0%)MRD阴性(MRD < 0.01%)。中位随访60.7(6.6~153.4)个月,中位无进展生存期为61.7(95%CI57.7~106.9)个月,中位总生存期未达到。Cox回归分析发现,患者血清β2-微球蛋白>3.5 mg/L和伴有TP53异常是影响无进展生存期的独立不良预后因素(P< 0.05),未发现对总生存期有意义的影响因素。单因素分析发现,治疗前IgA缺乏患者的总生存期显著缩短(P< 0.05);治疗后达骨髓MRD阴性患者的无进展生存期显著延长(未达到vs 35.6个月,P=0.016)。治疗后7例(20.0%)发生≥3级感染,15例(42.9%)发生≥3级血液学不良反应,除1例发生5级不良反应,其余不良反应均可恢复。结论 利妥昔单抗减量的FCR方案一线治疗CLL患者的总反应率、MRD转阴率及远期生存获益较理想,安全性可控。患者治疗前血清β2-微球蛋白>3.5 mg/L和伴有TP53异常影响无进展生存期,治疗前IgA缺乏可能影响总生存期;治疗后达骨髓MRD阴性的患者更易获得长期无进展生存。Abstract: Objective To investigate the efficacy and safety of the FCR regimen(fludarabine, cyclophosphamide and rituximab) with dose reduction of rituximab(375 mg/m2fixed dosage) in treatment-naive patients with chronic lymphocytic leukemia(CLL).Methods The clinical data of 35 CLL patients treated with frontline rituximab-reduced FCR regimen in our hospital from September 2009 to June 2021 were collected and analyzed.Results The median age before treatment initiation was 58(42-75) years in 35 patients, with 25 males and 10 females. The median treatment course was 6(2-6). The overall response rate was 91.4%(32/35), 15 cases(42.9%) achieved complete response, 3 cases(8.6%) achieved complete response with incomplete marrow recovery, 14 cases(40.0%) achieved partial response, 2 cases(5.7%) were in stable disease and 1 case(2.9%) had progressive disease. The bone marrow measurable residual disease(MRD) was detected in 20 patients, of which 14 cases(70.0%) achieved MRD negative(MRD < 0.01%). The median follow-up time was 60.7(6.6-153.4) months, the median progression-free survival was 61.7(95%CI57.7-106.9) months, and median overall survival was not reached. Cox regression analysis showed that serum β2-microglobulin>3.5 mg/L and TP53 abnormalities before treatment initiation were independent prognostic factors of progression-free survival(P< 0.05). No independent prognostic factors of overall survival were identified, but IgA deficiency was significantly correlated with shorter overall survival in univariate analysis(P< 0.05). Patients achieved bone marrow MRD negative post-treatment had a significant prolongation in progression-free survival(not reached vs 35.6 months,P=0.016). Seven cases(20.0%) experienced ≥grade 3 infection post-treatment and 15 cases(42.9%) experienced ≥grade 3 hematologic adverse events. All adverse events were resolved and recovered other than 1 case of grade 5 adverse events.Conclusion FCR regimen with does reduction of rituximab results in a high response rate and undetectable MRD rate with good long-term survival, and toxicities are acceptable, as frontline treatment for young CLL patients. Serum β2-microglobulin>3.5 mg/L and TP53 abnormalities before treatment initiation are independent prognostic factors of progression-free survival, and IgA level before treatment may have an impact on overall survival. To achieve bone marrow MRD negative post-treatment results in prolongation of progression-free survival.
-
Key words:
- chronic lymphocytic leukemia /
- fludarabine /
- cyclophosphamide /
- rituximab /
- drug tapering
-

-
表 1 影响患者PFS的预后因素分析
变量 单因素 多因素 χ2 P HR 95% CI P Binet A期vs B~C期(1 vs 34) 1.189 0.276 Rai Ⅰ期vs Ⅱ~Ⅳ期(11 vs 24) 1.176 0.278 β2-微球蛋白≤3.5 mg/L vs > 3.5 mg/L(22 vs 7) 6.064 0.014 5.778 1.292~25.837 0.022 TP53正常vs异常(20 vs 4) 5.030 0.025 6.056 1.307~28.050 0.021 治疗前IgA正常vs缺乏(16 vs 19) 0.829 0.362 表 2 影响患者OS的预后因素分析
变量 单因素 χ2 P Binet A期vs B~C期(1 vs 34) 0.316 0.574 Rai Ⅰ期vs Ⅱ~Ⅳ期(11 vs 24) 5.057 0.025 β2-微球蛋白≤3.5 mg/L vs > 3.5 mg/L(22 vs 7) 0.964 0.326 TP53正常vs异常(20 vs 4) 0.788 0.375 治疗前IgA正常vs缺乏(16 vs 19) 6.204 0.013 -
[1] Hallek M, Shanafelt TD, Eichhorst B. Chronic lymphocytic leukaemia[J]. Lancet, 2018, 391(10129): 1524-1537. doi: 10.1016/S0140-6736(18)30422-7
[2] 中华医学会血液学分会白血病淋巴瘤学组, 中国抗癌协会血液肿瘤专业委员会, 中国慢性淋巴细胞白血病工作组. 中国慢性淋巴细胞白血病/小淋巴细胞淋巴瘤的诊断与治疗指南(2018年版)[J]. 中华血液学杂志, 2018, 39(5): 353-358.
[3] Wierda WG, Rawstron A, Cymbalista F, et al. Measurable residual disease in chronic lymphocytic leukemia: expert review and consensus recommendations[J]. Leukemia, 2021, 35(11): 3059-3072. doi: 10.1038/s41375-021-01241-1
[4] Fischer K, Bahlo J, Fink AM, et al. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial[J]. Blood, 2016, 127(2): 208-215. doi: 10.1182/blood-2015-06-651125
[5] Herishanu Y, Tadmor T, Braester A, et al. Low-dose fludarabine and cyclophosphamide combined with standard dose rituximab(LD-FCR)is an effective and safe regimen for elderly untreated patients with chronic lymphocytic leukemia: The Israeli CLL study group experience[J]. Hematol Oncol, 2019, 37(2): 185-192. doi: 10.1002/hon.2580
[6] Kovacs G, Bahlo J, Kluth S, et al. Prognostic Impact and Risk Factors of Reducing Prescribed Doses of Fludarabine, Cyclophosphamide and Rituximab(FCR)during Frontline Treatment of Chronic Lymphocytic Leukemia(CLL)[J]. Blood, 2015, 126(23): 4156. doi: 10.1182/blood.V126.23.4156.4156
[7] Bouvet E, Borel C, Obéric L, et al. Impact of dose intensity on outcome of fludarabine, cyclophosphamide, and rituximab regimen given in the first-line therapy for chronic lymphocytic leukemia[J]. Haematologica, 2013, 98(1): 65-70. doi: 10.3324/haematol.2012.070755
[8] 王婷玉, 易树华, 王轶, 等. 氟达拉滨和环磷酰胺联合利妥昔单抗(FCR方案)一线治疗慢性淋巴细胞白血病43例临床分析[J]. 中华血液学杂志, 2021, 42(7): 543-548. https://cdmd.cnki.com.cn/Article/CDMD-10366-1018122862.htm
[9] Molica S, Giannarelli D, Mirabelli R, et al. Chronic lymphocytic leukemia international prognostic index(CLL-IPI)in patients receiving chemoimmuno or targeted therapy: a systematic review and meta-analysis[J]. Ann Hematol, 2018, 97(10): 2005-2008. doi: 10.1007/s00277-018-3350-5
[10] 李姮, 王婷玉, 尹乐, 等. 慢性淋巴细胞白血病免疫球蛋白重链可变区突变状态、基因片段使用特征及对预后的影响[J]. 中华血液学杂志, 2021, 42(12): 1025-1029.
[11] 李晓彤, 朱华渊, 王莉, 等. 传统免疫化疗时代118例TP53基因异常慢性淋巴细胞白血病患者生存分析[J]. 中华血液学杂志, 2019, 40(5): 378-383.
[12] Allan JN, Shanafelt T, Wiestner A, et al. Long-term efficacy of first-line ibrutinib treatment for chronic lymphocytic leukaemia in patients with TP53 aberrations: a pooled analysis from four clinical trials[J]. Br J Haematol, 2022, 196(4): 947-953. doi: 10.1111/bjh.17984
[13] Visentin A, Mauro FR, Cibien F, et al. Continuous treatment with Ibrutinib in 100 untreated patients with TP53 disrupted chronic lymphocytic leukemia: A real-life campus CLL study[J]. Am J Hematol, 2022, 97(3): E95-E99.
[14] 沈晖, 朱华渊, 李建勇. BCL-2抑制剂在慢性淋巴细胞白血病中的研究进展[J]. 临床血液学杂志, 2022, 35(1): 77-81. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXZ202201015.htm
[15] Vitale C, Boccellato E, Comba L, et al. Impact of Immune Parameters and Immune Dysfunctions on the Prognosis of Patients with Chronic Lymphocytic Leukemia[J]. Cancers(Basel), 2021, 13(15): 3856.
[16] Smolej L. Incidence and prognostic significance of serum immunoglobulins and paraproteins in patients with chronic lymphocytic leukaemia: another valuable piece of the puzzle[J]. Br J Haematol, 2020, 190(6): 815-816. doi: 10.1111/bjh.16972
[17] Parikh SA, Leis JF, Chaffee KG, et al. Hypogammaglobulinemia in newly diagnosed chronic lymphocytic leukemia: Natural history, clinical correlates, and outcomes[J]. Cancer, 2015, 121(17): 2883-2891. doi: 10.1002/cncr.29438
[18] Mauro FR, Morabito F, Vincelli ID, et al. Clinical relevance of hypogammaglobulinemia, clinical and biologic variables on the infection risk and outcome of patients with stage A chronic lymphocytic leukemia[J]. Leuk Res, 2017, 57: 65-71. doi: 10.1016/j.leukres.2017.02.011
[19] Reda G, Cassin R, Gentile M, et al. IgA hypogammaglobulinemia predicts outcome in chronic lymphocytic leukemia[J]. Leukemia, 2019, 33(6): 1519-1522. doi: 10.1038/s41375-018-0344-1
[20] Ishdorj G, Streu E, Lambert P, et al. IgA levels at diagnosis predict for infections, time to treatment, and survival in chronic lymphocytic leukemia[J]. Blood Adv, 2019, 3(14): 2188-2198. doi: 10.1182/bloodadvances.2018026591
[21] Corbingi A, Innocenti I, Tomasso A, et al. Monoclonal gammopathy and serum immunoglobulin levels as prognostic factors in chronic lymphocytic leukaemia[J]. Br J Haematol, 2020, 190(6): 901-908. doi: 10.1111/bjh.16975
-




 下载:
下载: