Factors affecting the relapse of non-severe aplastic anemia treated with cyclosporine A
-
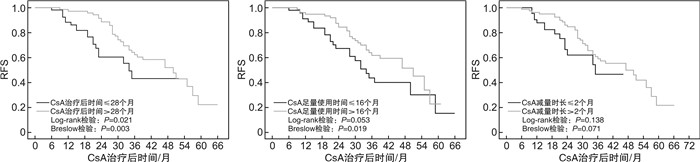
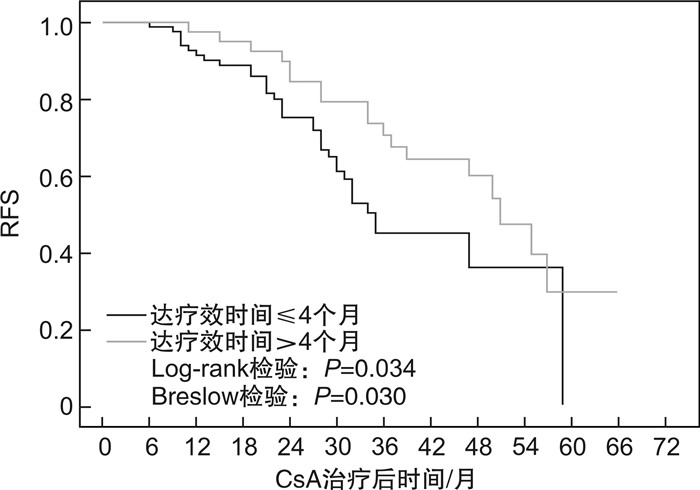
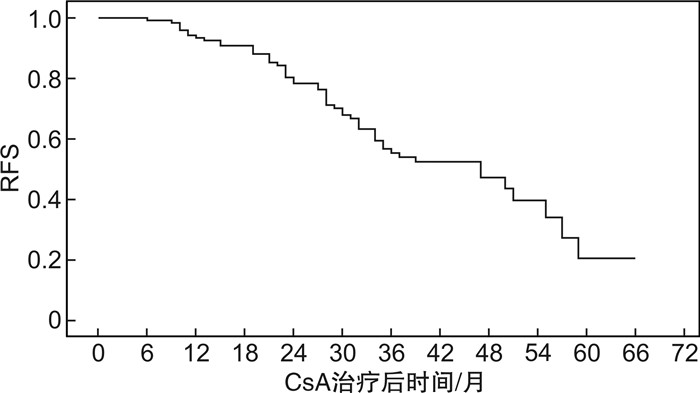
摘要: 目的分析环孢霉素A(CsA)单药治疗的非重型再生障碍性贫血(NSAA)患者获得疗效后,影响复发的因素。方法回顾性收集2012年10月—2020年11月在北京协和医院就诊并采用标准剂量CsA单药治疗的初治NSAA患者资料,分析获得疗效者基线特征、CsA治疗持续时间、足量使用时间、减量时长等因素对其无复发生存(RFS)的影响。结果共纳入179例患者,男性占45.3%,中位年龄46(14~85)岁。中位CsA使用时间24(6~80)个月。69.3%的患者获得疗效,中位起效时间3(1~39)个月。在中位随访34(8~101)个月内,42.7%患者复发,中位复发时间28(6~59)个月,中位RFS为47个月。单因素分析显示,基线中性粒细胞(Neu)高、CsA治疗持续时间>28个月、CsA足量使用时间>16个月、起效时间>4个月者RFS率更高(P=0.006,0.021,0.019,0.034);多因素Cox回归分析发现,基线Neu高(P=0.006,HR=1.144)及CsA治疗持续时间>28个月(P=0.019,HR=2.045)是RFS的独立影响因素。结论CsA单药治疗的初治NSAA患者,其初始较高的Neu水平、较长的CsA治疗时间等可能与较低的复发率相关。Abstract: ObjectiveTo analyze the risk factors of relapse after effective treatment by cyclosporine A(CsA) in patients with newly diagnosed non-severe aplastic anemia(NSAA).MethodsData of patients with newly diagnosed NSAA receiving standard-dose CsA monotherapy at Peking Union Medical College Hospital from October 2012 to November 2020 were reviewed. The correlation between relapse-free survival(RFS) and risk factors such as patients' baseline characteristics, total CsA duration, full-dosage CsA duration and CsA taper was analyzed.ResultsA total of 179 patients were enrolled, with 81(45.3%) of males and a median age of 46(14-85) years. The median CsA duration was 24(6-80) months, with 69.3% responded at a median time of 3(1-39) months. During 34(8-101) months' follow-up, 42.7% patients relapsed at a median of 28(6-59) months, making median RFS 47 months. Univariate analysis demonstrated that increased baseline neutrophil count, total CsA duration>28 months, full-dosage CsA duration>16 months and time to response>4 months correlated with higher RFS(P=0.006, 0.021, 0.019 and 0.034, respectively). Multivariate Cox regression analysis showed that only increased baseline neutrophil count(P=0.006, HR=1.144) and total CsA duration>28 months(P=0.019, HR=2.045) were independent risk factors of RFS.ConclusionIncreased level of baseline neutrophils and longer CsA duration may predict lower relapse rate in patients with newly diagnosed NSAA treated with CsA monotherapy.
-
Key words:
- aplastic anemia /
- cyclosporine A /
- relapse /
- neutrophil count /
- cyclosporine A treatment duration
-

-
表 1 NSAA患者基线临床特征
临床特征 所有患者(n=179) 年龄/岁 47.0(14.0~85.0) 男:女/例 81:98 Hb/(g·L-1) 84.0(25.0~173.0) Ret/(×109·L-1) 6.3(2.7~10.9) Neu/(×109·L-1) 1.2 (0~7.4) PLT/(×109·L-1) 27.0(2.0~87.0) Fer/(ng·mL-1) 386.5(56.1~7 811.5) ALT/(U·L-1) 17.0(3.0~60.0) SCr/(μmol·L-1) 64.5(37.2~115.3) Dbil/(μmol·L-1) 3.9(1.0~10.1) 表 2 CsA起效者基线临床特征与RFS关系
临床特征 获得疗效者(n=124) P 年龄/岁 47.5(14.0~85.0) 0.703 男∶女/例 48∶76 0.436 随访时间/月 38(8~101) 0.434 Hb/(g·L-1) 82(25~163) 0.997 Ret/(×109·L-1) 6.8(2.9~10.9) 0.991 Neu/(×109·L-1) 1.2 (0~7.2) 0.006 PLT/(×109·L-1) 24.5(2.0~81.0) 0.566 Fer/(ng·mL-1) 372(56~2381) 0.662 ALT/(U·L-1) 17(3~58) 0.626 SCr/(μmol·L-1) 63(37~115) 0.879 Dbil/(μmol·L-1) 3.7(1.4~10.1) 0.436 PNH克隆/例 15/84 0.078 染色体及基因异常/例 11/21 0.763 注:分类变量P值由log-rank检验比较组间的RFS差异得出;连续变量P值由单因素Cox回归分析其对RFS的影响显著性得出。 表 3 CsA治疗持续时间、足量使用时间、减量时长与RFS关系
类别 获得疗效者
(n=124)P CsA治疗持续时间 30(6~80) 0.177 CsA治疗持续时间≤28个月 54.0(43.5) 0.021a) CsA治疗持续时间>28个月 70.0(56.5) 0.003b) CsA足量使用时间 19(2~59) 0.607 CsA足量使用时间≤16个月 48.0(38.7) 0.053a) CsA足量使用时间>16个月 76.0(61.3) 0.019b) CsA减量时长c) 8(0~73) 0.352 CsA减量时长≤2个月 45.0(36.3) 0.138a) CsA减量时长>2个月 79.0(63.7) 0.071b) 注:P值由RFS影响因素的单因素Cox回归分析得出。a)log-rank检验比较;b)Breslow(Generalized Wilcoxon)检验比较;c)CsA未减量记为CsA减量时长=0,纳入CsA减量时长≤2个月组。 -
[1] Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia[J]. Br J Haematol, 2016, 172(2): 187-207. doi: 10.1111/bjh.13853
[2] Brzez ' niakiewicz-Janus K, Rupa-Matysek J, Gil L. Acquired Aplastic Anemia as a Clonal Disorder of Hematopoietic Stem Cells[J]. Stem Cell Rev Rep, 2020, 16(3): 472-481. doi: 10.1007/s12015-020-09971-y
[3] Dufour C, Svahn J, Bacigalupo A, et al. Front-line immunosuppressive treatment of acquired aplastic anemia[J]. Bone Marrow Transplant, 2013, 48(2): 174-177. doi: 10.1038/bmt.2012.222
[4] 李瑞鑫, 金媛媛, 杨岩, 等. 艾曲泊帕联合强化免疫抑制疗法治疗成人重型再生障碍性贫血疗效的预测因素[J]. 临床血液学杂志, 2022, 35(5): 333-337. http://www.whuhzzs.com/article/doi/10.13201/j.issn.1004-2806.2022.05.007
[5] Scheinberg P, Rios O, Scheinberg P, et al. Prolonged cyclosporine administration after antithymocyte globulin delays but does not prevent relapse in severe aplastic anemia[J]. Am J Hematol, 2014, 89(6): 571-574. doi: 10.1002/ajh.23692
[6] Matsuda K, Koya J, Arai S, et al. Cyclosporine Therapy in Patients with Transfusion-independent Non-severe Aplastic Anemia: A Retrospective Analysis[J]. Intern Med, 2019, 58(3): 355-360. doi: 10.2169/internalmedicine.1372-18
[7] Patel BJ, Barot SV, Kuzmanovic T, et al. Distinctive and common features of moderate aplastic anaemia[J]. Br J Haematol, 2020, 189(5): 967-975. doi: 10.1111/bjh.16460
[8] 宋琳, 赵馨, 彭广新, 等. R-ATG联合环孢素与环孢素联合雄激素一线治疗输血依赖非重型再生障碍性贫血的疗效比较: 单中心回顾性研究[J]. 临床血液学杂志, 2019, 32(5): 358-362, 366. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXZ201905008.htm
[9] Zhang XT, Wang X, Cao J, et al. Treatment outcome of 301 aplastic anemia patients in China: a 10-year follow-up and real-world data from single institute experience[J]. Hematology, 2021, 26(1): 1025-1030.
[10] Zhu XF, He HL, Wang SQ, et al. Current Treatment Patterns of Aplastic Anemia in China: A Prospective Cohort Registry Study[J]. Acta Haematol, 2019, 142(3): 162-170. doi: 10.1159/000499065
[11] 卢文婕, 张琳, 杨李, 等. 环孢素A治疗儿童非重型再生障碍性贫血的转归效果及预后因素分析[J]. 中国实验血液学杂志, 2021, 29(4): 1257-1261. https://www.cnki.com.cn/Article/CJFDTOTAL-XYSY202104039.htm
[12] 张梦露, 陈婉淑, 韩冰. 重组人血小板生成素对非重型再生障碍性贫血免疫抑制治疗疗效的影响[J]. 中华血液学杂志, 2020, 41 (8): 637-642. https://www.cnki.com.cn/Article/CJFDTOTAL-HKHT202001015.htm
[13] Jalaeikhoo H, Khajeh-Mehrizi A. Immunosuppressive therapy in patients with aplastic anemia: a single-center retrospective study[J]. PLoS One, 2015, 10(5): e0126925. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4430492/
[14] Li H, Fu L, Yang B, et al. Cyclosporine Monotherapy in Pediatric Patients With Non-severe Aplastic Anemia: A Retrospective Analysis[J]. Front Med(Lausanne), 2022, 9: 805197.
[15] 刘晨曦, 宋琳, 张莉, 等. 环孢素A联合雄激素治疗输血依赖非重型再生障碍性贫血预后因素分析[J]. 中华血液学杂志, 2020, 41 (3): 234-238.
[16] Kamio T, Ito E, Ohara A, et al. Relapse of aplastic anemia in children after immunosuppressive therapy: a report from the Japan Childhood Aplastic Anemia Study Group[J]. Haematologica, 2011, 96(6): 814-819.
[17] Boddu P, Garcia-Manero G, Ravandi F, et al. Clinical outcomes in adult patients with aplastic anemia: A single institution experience[J]. Am J Hematol, 2017, 92(12): 1295-1302. https://onlinelibrary.wiley.com/doi/full/10.1002/gcc.20793
[18] Schrezenmeier H, Marin P, Raghavachar A, et al. Relapse of aplastic anaemia after immunosuppressive treatment: a report from the European Bone Marrow Transplantation Group SAA Working Party[J]. Br J Haematol, 1993, 85(2): 371-377. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-2141.1993.tb03181.x
[19] Saracco P, Quarello P, Iori AP, et al. Cyclosporin A response and dependence in children with acquired aplastic anaemia: a multicentre retrospective study with long-term observation follow-up[J]. Br J Haematol, 2008, 140(2): 197-205.
[20] 付蓉. 再生障碍性贫血诊断与治疗中国专家共识(2017年版)[J]. 中华血液学杂志, 2017, 38(1): 1-5. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXZ201711001.htm
[21] Camitta BM, Rappeport JM, Parkman R, et al. Selection of patients for bone marrow transplantation in severe aplastic anemia[J]. Blood, 1975, 45(3): 355-363.
[22] Groarke EM, Patel BA, Gutierrez-Rodrigues F, et al. Eltrombopag added to immunosuppression for children with treatment-naïve severe aplastic anaemia[J]. Br J Haematol, 2021, 192(3): 605-614.
[23] Bacigalupo A, Bruno B, Saracco P, et al. Antilymphocyte globulin, cyclosporine, prednisolone, and granulocyte colony-stimulating factor for severe aplastic anemia: an update of the GITMO/EBMT study on 100 patients. European Group for Blood and Marrow Transplantation(EBMT)Working Party on Severe Aplastic Anemia and the Gruppo Italiano Trapianti di Midolio Osseo(GITMO)[J]. Blood, 2000, 95(6): 1931-1934.
[24] Dinçol G, Aktan M, Diz-Küçükkaya R, et al. Treatment of acquired severe aplastic anemia with antilymphocyte globulin, cyclosporin A, methyprednisolone, and granulocyte colony-stimulating factor[J]. Am J Hematol, 2007, 82(9): 783-786. https://onlinelibrary.wiley.com/doi/abs/10.1002/ajh.20954
[25] Patel BA, Groarke EM, Lotter J, et al. Long-term outcomes in patients with severe aplastic anemia treated with immunosuppression and eltrombopag: a phase 2 study[J]. Blood, 2022, 139(1): 34-43.
-




 下载:
下载: