Clinical utility of CD4 and CD8 T-lymphocytes ATP levels in patients following allogeneic hematopoietic stem cell transplantation
-
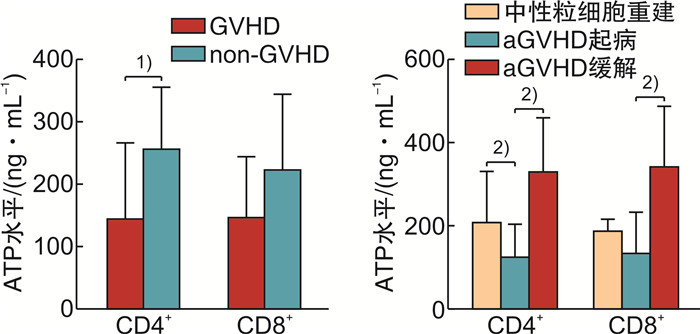
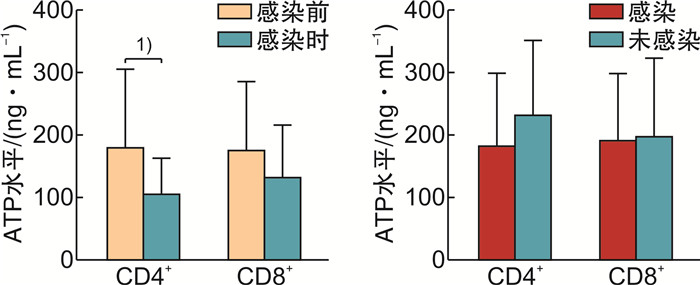
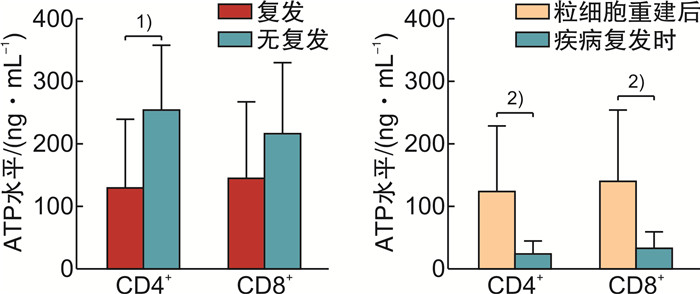
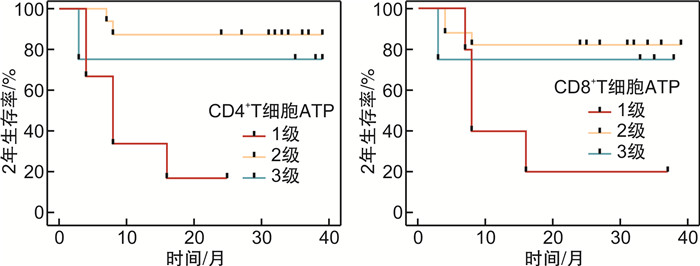
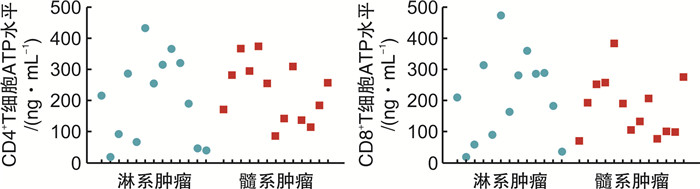
摘要: 目的探讨荧光素酶法检测CD4+和CD8+T淋巴细胞三磷酸腺苷(ATP)水平在异基因造血干细胞移植(allo-HSCT)后感染、急性移植物抗宿主病(aGVHD)及疾病复发的变化和临床意义。方法纳入2019年1月—2020年5月接受allo-HSCT的血液系统肿瘤患者为研究对象,荧光素酶法检测allo-HSCT后不同时间点患者的CD4+和CD8+T细胞ATP水平,分析其与aGVHD、感染和肿瘤复发的关系。结果纳入本研究患者26例,中位年龄31(17~64)岁。allo-HSCT后ATPCD4和ATPCD8分别为(217.50 ± 117.59)ng/mL和(196.23 ± 117.32)ng/mL,均比健康人群低(P < 0.05)。发生aGVHD的中位时间为+48(+25~+184) d,发生Ⅲ~Ⅳ度aGVHD患者基础ATPCD4比轻度和未发生GVHD的患者明显下降(P=0.018),而且,aGVHD时的ATPCD4比粒细胞重建时更低,aGVHD缓解后ATPCD4和ATPCD8均比起病时明显回升(P < 0.01)。allo-HSCT后感染的中位时间为+67(+36~+218) d。感染后平均ATPCD4比感染前明显下降(P=0.047),基础ATP水平在+67 d内感染和非感染患者间差异无统计学意义。总体中位随访时间为31(3~39)个月,2年内复发患者8例(30.77%),死亡患者8例(30.77%)。复发患者基础ATPCD4比无复发患者明显下降(P=0.009),基础ATPCD4≤ 99.90 ng/mL是死亡的独立危险因素(P=0.009)。结论ATPCD4对预测Ⅲ~Ⅳ度严重GVHD和疾病复发及不良预后有一定价值。Abstract: ObjectiveTo evaluate the clinical utility of CD4 and CD8 T-lymphocytes adenosine triphosphate(ATP) levels in predicting infection, acute GVHD(aGVHD) and disease relapse after allogeneic hematopoietic stem cell transplantation(allo-HSCT).MethodsPatients with hematology malignancy who underwent allo-HSCT between January 2019 to May 2020 were retrospectively studied. Luminescent ATP detection assay was used to assess CD4 and CD8 ATP levels which were compared at different time points when patients had neutrophil reconstitution, aGVHD onset, infection and disease relapse.ResultsA total of 26 patients were included with the median age of 31 years(range 17-64 years). CD4 and CD8 ATP levels at the time of neutrophil reconstitution were(217.50 ± 117.59)ng/mL and (196.23 ± 117.32)ng/mL, respectively, which were much lower than those of healthy people(P < 0.05). Patients developed aGVHD at a median time of +48 d(range +25 to +184 days). Initial CD4 ATP levels of patients with grade Ⅲ-Ⅳ aGVHD were significantly lower than those with gradeⅠ-Ⅱor without aGVHD(P=0.018). CD4 ATP levels during aGVHD were lower than initial levels but increased as aGVHD improved(P < 0.01). Patients had infection at a median time of +67 d(range +36 to +218 days). CD4 ATP levels during infection were lower than initial levels which showed no difference between patients with or without infection within +67 d post allo-HSCT. Patients were followed up for 3 to 39 months with a median follow-up of 31 months and 8 patients (30.77%) relapsed and 8 patients (30.77%) died within 2 years. CD4 ATP levels at relapse was dramatically decreased and initial CD4 ATP levels of ≤ 99.90 ng/mL was confirmed to be a risk factor for poor clinical outcome(P=0.009).ConclusionAssessment of CD4 and CD8 ATP levels by Luminescent ATP Detection Assay Kit play an important role in predicting grade Ⅲ-Ⅳ aGVHD and disease relapse after allo-HSCT.
-

-
表 1 ATPCD4和ATPCD8在中性粒细胞重建时、aGVHD发生时和aGVHD缓解后的比较
X±S 时间 ATPCD4/(ng·mL-1) ATPCD8/(ng·mL-1) 淋巴细胞/(×109·L-1) Th/Ts CsA/(ng·mL-1) 粒细胞重建 208.85±120.49 188.25±26.98 1.26±0.55 0.47±0.20 213.84±98.65 aGVHD起病 126.05±77.08 135.95±95.86 1.01±0.56 0.56±0.24 215.22±81.61 aGVHD缓解 330.67±126.30 343.33±141.15 1.43±0.43 0.48±0.10 246.01±94.11 P < 0.01 < 0.01 0.066 0.286 0.528 -
[1] Iftikhar R, Chaudhry Q, Anwer F, et al. Allogeneic hematopoietic stem cell transplantation in aplastic anemia: current indications and transplant strategies[J]. Blood Rev, 2021, 47: 100772. doi: 10.1016/j.blre.2020.100772
[2] Barriga F, Ramírez P, Wietstruck A, et al. Hematopoietic stem cell transplantation: clinical use and perspectives[J]. Biol Res, 2012, 45(3): 307-316. doi: 10.4067/S0716-97602012000300012
[3] Ferrara JL, Levine JE, Reddy P, et al. Graft-versus-host disease[J]. Lancet, 2009, 373(9674): 1550-1561. doi: 10.1016/S0140-6736(09)60237-3
[4] Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy[J]. Nat Rev Immunol, 2012, 12(6): 443-458. https://www.nature.com/articles/nri3212/
[5] 李娜, 周阳, 赵晨, 等. 单倍型造血干细胞移植与同胞相合造血干细胞移植治疗慢性粒-单核细胞白血病的疗效比较[J]. 临床血液学杂志, 2022, 35(5): 323-327. http://www.whuhzzs.com/article/doi/10.13201/j.issn.1004-2806.2022.05.005
[6] Di Bartolomeo P, Santarone S, De Angelis G, et al. Haploidentical, unmanipulated, G-CSF-primed bone marrow transplantation for patients with high-risk hematologic malignancies[J]. Blood, 2013, 121(5): 849-857. doi: 10.1182/blood-2012-08-453399
[7] Ogonek J, Kralj Juric M, Ghimire S, et al. Immune Reconstitution after Allogeneic Hematopoietic Stem Cell Transplantation[J]. Front Immunol, 2016, 7: 507. https://www.frontiersin.org/articles/10.3389/fimmu.2016.00507/full
[8] Velardi E, Tsai JJ, van den Brink M. T cell regeneration after immunological injury[J]. Nat Rev Immunol, 2021, 21(5): 277-291. doi: 10.1038/s41577-020-00457-z
[9] Gordon MY, Blackett NM. Reconstruction of the hematopoietic system after stem cell transplantation[J]. Cell Transplant, 1998, 7(4): 339-344. doi: 10.1177/096368979800700401
[10] 中华医学会血液学分会, 中国医师协会血液科医师分会. 中国中性粒细胞缺乏伴发热患者抗菌药物临床应用指南(2020年版)[J]. 中华血液学杂志, 2020, 41(12): 969-978. doi: 10.3760/cma.j.issn.0253-2727.2020.12.001
[11] Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade[J]. Br J Haematol, 1997, 97(4): 855-864. doi: 10.1046/j.1365-2141.1997.1112925.x
[12] Schoemans HM, Lee SJ, Ferrara JL, et al. EBMT-NIH-CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment[J]. Bone Marrow Transplant, 2018, 53(11): 1401-1415. doi: 10.1038/s41409-018-0204-7
[13] 中华医学会血液学分会白血病淋巴瘤学组. 中国成人急性髓系白血病(非急性早幼粒细胞白血病)诊疗指南(2021年版)[J]. 中华血液学杂志, 2021, 42(8): 617-623.
[14] 中国抗癌协会血液肿瘤专业委员会, 中华医学会血液学分会白血病淋巴瘤学组. 中国成人急性淋巴细胞白血病诊断与治疗指南(2021年版)[J]. 中华血液学杂志, 2021, 42(9): 705-716. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXZ202203002.htm
[15] Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification[J]. J Clin Oncol, 2014, 32(27): 3059-3068. doi: 10.1200/JCO.2013.54.8800
[16] Gesundheit B, Budowski E, Israeli M, et al. Assessment of CD4 T-lymphocyte reactivity by the Cylex ImmuKnow assay in patients following allogeneic hematopoietic SCT[J]. Bone Marrow Transplant, 2010, 45(3): 527-533. doi: 10.1038/bmt.2009.182
[17] Lee LE, Barsoumian AE, Brown AW, et al. Rates of Microbiologically Diagnosed Infection and Pathogen Detection in Hematopoietic Stem Cell Transplant Patients[J]. Mil Med, 2016, 181(11): e1685-e1691. doi: 10.7205/MILMED-D-15-00553
[18] Omrani AS, Almaghrabi RS. Complications of hematopoietic stem cell transplantation: Bacterial infections[J]. Hematol Oncol Stem Cell Ther, 2017, 10(4): 228-232. doi: 10.1016/j.hemonc.2017.05.018
[19] 杨飞燕, 谢春红, 陈磊, 等. 芦可替尼治疗激素难治性移植物抗宿主病有效性和安全性的meta分析[J]. 临床血液学杂志, 2022, 35(5): 369-374. http://www.whuhzzs.com/article/doi/10.13201/j.issn.1004-2806.2022.05.014
[20] Barten MJ, Tarnok A, Garbade J, et al. Pharmacodynamics of T-cell function for monitoring immunosuppression[J]. Cell Prolif, 2007, 40(1): 50-63.
[21] Robb RJ, Kreijveld E, Kuns RD, et al. Type I-IFNs control GVHD and GVL responses after transplantation[J]. Blood, 2011, 118(12): 3399-409. https://research.monash.edu/en/publications/type-i-ifns-control-gvhd-and-gvl-responses-after-transplantation
[22] Pirogova OV, Moiseev IS, Surkova EA, et al. Profiles of pro-inflammatory cytokines in allogenic stem cell transplantation with post-transplant cyclophosphamide[J]. Cytokine, 2017, 99: 148-153. https://www.sciencedirect.com/science/article/pii/S1043466617302491
[23] Bhorade SM, Janata K, Vigneswaran WT, et al. Cylex ImmuKnow assay levels are lower in lung transplant recipients with infection[J]. J Heart Lung Transplant, 2008, 27(9): 990-994. https://www.sciencedirect.com/science/article/pii/S1053249808004774
[24] Moon HH, Kim TS, Roh YN, et al. Can immune function assay predict infection or recovery?[J]. Transplant Proc, 2012, 44(4): 1048-1051. https://www.sciencedirect.com/science/article/pii/S0041134512003685
[25] Moon HH, Kim TS, Lee S, et al. Serial ImmuKnow assay in stable kidney transplant recipients[J]. Cent Eur J Immunol, 2014, 39(1): 96-99. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4439976/
[26] Piloni D, Magni S, Oggionni T, et al. Clinical utility of CD4+ function assessment(ViraCor-IBT ImmuKnow test)in lung recipients[J]. Transpl Immunol, 2016, 37: 35-39. https://www.sciencedirect.com/science/article/pii/S0966327416300168
[27] Weston MW, Rinde-Hoffman D, Lopez-Cepero M. Monitoring cell-mediated immunity during immunosuppression reduction in heart transplant recipients with severe systemic infections[J]. Clin Transplant, 2020, 34(3): e13809.
[28] Ravaioli M, Neri F, Lazzarotto T, et al. Immunosuppression Modifications Based on an Immune Response Assay: Results of a Randomized, Controlled Trial[J]. Transplantation, 2015, 99(8): 1625-1632.
[29] Berglund D, Bengtsson M, Biglarnia A, et al. Screening of mortality in transplant patients using an assay for immune function[J]. Transpl Immunol, 2011, 24(4): 246-250. https://www.sciencedirect.com/science/article/pii/S0966327411000025
[30] Huskey J, Gralla J, Wiseman AC. Single time point immune function assay(ImmuKnow)testing does not aid in the prediction of future opportunistic infections or acute rejection[J]. Clin J Am Soc Nephrol, 2011, 6(2): 423-429.
[31] Israeli M, Klein T, Herscovici C, et al. Cellular immune function monitoring after allogeneic haematopoietic cell transplantation: evaluation of a new assay[J]. Clin Exp Immunol, 2013, 172(3): 475-482. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3646447/
[32] Edinger M, Hoffmann P, Ermann J, et al. CD4+CD25+regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation[J]. Nat Med, 2003, 9(9): 1144-1150. https://www.nature.com/articles/nm915
[33] Mehrotra A, Leventhal J, Purroy C, et al. Monitoring T cell alloreactivity[J]. Transplant Rev(Orlando), 2015, 29(2): 53-59. https://www.sciencedirect.com/science/article/pii/S0955470X14000858
-




 下载:
下载: