Clinical features of bendamustine combined with rituximab in the treatment of advanced marginal zone lymphoma
-
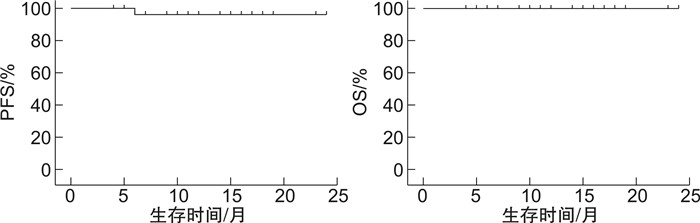
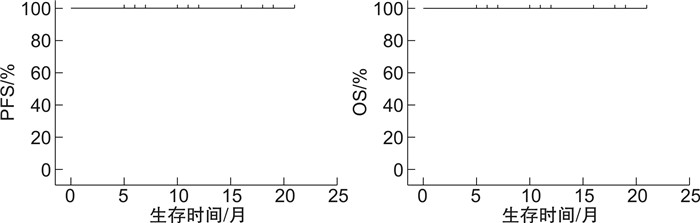
摘要: 目的研究影响苯达莫司汀联合利妥昔单抗(BR)治疗边缘区淋巴瘤(MZL)疗效的相关临床因素和分子特征。方法回顾性分析2020年3月—2021年9月上海交通大学医学院附属瑞金医院血液科收治的48例MZL患者的临床资料,初治患者29例,复发/进展患者19例,均应用BR方案(利妥昔单抗375 mg/m2,d0,苯达莫司汀90 mg/m2,d1~2)进行治疗。48例患者中有36例进行了DNA测序,以进一步探究基因突变对MZL患者中BR方案疗效的影响。结果48例患者中位年龄65.5(22.0~79.0)岁,男女比例1.5:1.0。29例初治患者中,20例达完全代谢学缓解(CMR),8例达部分代谢学缓解(PMR),CMR率69.0%(20/29),总有效率(ORR)96.6%(28/29)。19例复发/进展患者中,13例达CMR,4例达PMR,CMR率68.4%(13/19),ORR 89.5%(17/19)。中位随访12(6~24)个月,初治患者1年疾病无进展生存(PFS)率为96.2%,1年总生存(OS)率为100.0%;复发/进展患者1年PFS率为100.0%,1年OS率为100.0%。进一步分析发现,MZL的起源部位[黏膜相关淋巴组织MALT淋巴瘤(胃、肺、其他)、脾边缘区淋巴瘤、结内边缘区淋巴瘤]、既往是否接受过治疗、肿块直径>7.5 cm、多个结外器官受累、骨髓受累、IPI评分3~5分、β2微球蛋白(β2-MG)升高、免疫固定电泳阳性,FISH-MALT1阳性均对患者的CMR率无显著影响(P≥0.05)。36例患者的DNA测序结果显示,其中20例(20/36,55.6%)MZL患者至少检出一个基因突变。肿瘤基因突变通路对患者的CMR率无显著影响(P≥0.05)。安全性方面,血液学不良反应以中性粒细胞减少、血小板减少和贫血为主,非血液学不良反应以恶心、呕吐和皮疹为主。结论不论是初治还是复发/进展的MZL患者,BR方案均有确切的临床疗效,患者耐受性良好。本研究为单中心回顾性研究,还需要前瞻性大样本量的临床研究来验证。Abstract: ObjectiveTo investigate the clinical and genetic characteristics associated with bendamustine combined with rituximab(BR) in treating marginal zone lymphoma(MZL).MethodsThe clinical data of 48 MZL patients diagnosed from March 2020 to September 2021 were retrospectively evaluated, including 29 newly diagnosed patients and 19 relapsed or progressive patients. All patients were treated with BR regimen(rituximab 375 mg/m2 d0, bendamustine 90 mg/m2, d1-2). Targeted sequencing was applied in 36 patients to further explore the impact of genetic mutations on BR therapy in MZL patients.ResultsThe median age of the 48 patients was 65.5(22.0-79.0 years), and the male-to-female ratio was 1.5∶1.0. Among the 29 newly diagnosed patients, 20 patients achieved complete metabolic response(CMR) and 8 patients achieved partial metabolic response(PMR), the CMR rate was 69.0%(20/29) and the overall response rate(ORR) was 96.6%(28/29). Among 19 patients with relapsed or progressive diseases, 13 patients achieved CMR(68.4%, 13/19), 4 patients achieved PMR(21.1%, 4/19), and the ORR was 89.5%(17/19). With a median follow-up of 12(6-24) months, the 1-year progression-free survival(PFS) rate and 1-year OS rate of newly diagnosed patients were 96.2% and 100.0%, respectively. The 1-year PFS rate and 1-year OS rate of relapsed or progressive patients were 100.0%. Furthermore, the subtype of MZL(MALT lymphoma[gastric, lung, others], SMZL and NMZL), prior treatment, the bulky mass, multiple extranodal involvement, bone marrow involvement, advanced IPI score, positive immunofixation electrophoresis, positive FISH-MALT1 did not exert impacts on CMR rate of patients. DNA sequencing of 36 patients showed that at least one mutation was detected in 20 MZL patients(20/36, 55.6%). There was no significant relationship was observed between the oncogenic pathways enriched by tumor gene mutations and CMR in patients. BR regimen was well tolerated in MZL patients. In terms of safety, hematologic adverse events mainly included neutropenia, thrombocytopenia and anemia, while non-hematologic adverse events mainly including nausea, vomiting and rash.ConclusionBR regimen is efficacy and tolerable in both newly diagnosed and relapsed or progressive MZL patients. This is a single center retrospective study which needs to be validated by large scale perspective clinical trial.
-
Key words:
- marginal zone lymphoma /
- bendamustine /
- rituximab /
- gene mutation /
- prognosis
-

-
表 1 48例MZL患者临床特征
指标 例数(%) 指标 例数(%) 既往是否接受过治疗 LDH 是 19(39.6) 正常 39(81.2) 否 29(60.4) 升高 9(18.8) 性别 β2-MG 男 29(60.4) 正常 30(62.5) 女 19(39.6) 升高 18(37.5) 年龄/岁 肿块直径>7.5 cm >60 30(62.5) 有 21(43.8) ≤60 18(37.5) 无 27(56.2) 起源部位 骨髓受累 MALT淋巴瘤(肺) 17(35.4) 有 21(43.8) MALT淋巴瘤(胃) 5(10.4) 无 27(56.2) MALT淋巴瘤(其他) 5(10.4)ECOG评分/分 SMZL 14(29.2) 0~1 39(81.2) NMZL 7(14.6) 2~3 9(18.8) IPI评分/分 B症状 1~2 28(58.3) 有 6(12.5) 3~5 20(41.7) 无 42(87.5) 累及结外器官处/处 免疫固定电泳 0/1 20(41.7) 阳性 14(29.2) ≥2 28(58.3) 阴性 34(70.8) Hb FISH-MALT1 正常 20(41.7) 阳性 5(10.4) 降低 28(58.3) 阴性 43(89.6) 注:Hb降低: < 120 g/L;LDH升高:>192 U/L;β2-MG升高:>2366 ng/mL。 表 2 初治及复发/进展MZL患者基因突变频率比较
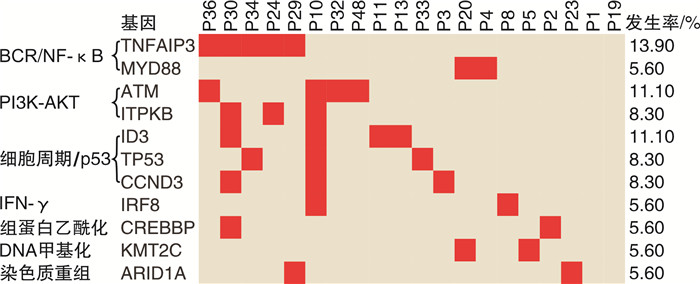
基因突变 突变频率/% P 初治 复发/进展 ATM 5.3 17.6 0.326 TNFAIP3 10.5 17.6 0.650 TP53 5.3 11.8 0.593 CREBBP 5.3 5.9 1.000 ITPKB 10.5 5.9 1.000 CCND3 10.5 5.9 1.000 ID3 15.8 5.9 0.605 MYD88 10.5 0 0.487 KMT2C 10.5 0 0.487 IRF8 10.5 0 0.487 ARID1A 10.5 0 0.487 表 3 影响MZL患者疗效的单因素分析
例(%) 影响因素 总例数
(n=48)CMR P 起源部位 0.271 MALT淋巴瘤(肺) 17 10(58.8) MALT淋巴瘤(胃) 5 4(80.0) MALT淋巴瘤(其他) 5 5(100.0) SMZL 14 8(57.1) NMZL 7 6(85.7) 既往是否接受过治疗 0.875 是 19 13(68.4) 否 29 20(69.0) 肿块直径>7.5 cm 0.367 是 21 13(61.9) 否 27 20(74.1) 结外器官受累/个 0.269 ≥2 28 20(71.4) < 2 20 13(65.0) 骨髓累及 0.724 是 21 15(71.4) 否 27 18(66.7) IPI评分/分 0.269 ≥3 20 12(60.0) < 3 28 21(75.0) β2-MG升高 1.000 是 18 12(66.7) 否 30 21(70.0) 免疫固定电泳阳性 0.797 是 14 10(71.4) 否 34 23(67.6) FISH-MALT1阳性 0.143 是 5 2(40.0) 否 43 31(75.6) 表 4 影响MZL患者疗效的基因分析
例(%) 信号通路 总例数
(n=36)CMR P BCR/NF-κB 0.681 有突变 7 5(71.4) 无突变 29 17(58.6) PI3K-AKT 0.063 有突变 6 6(100.0) 无突变 30 16(53.3) 细胞周期/p53 0.394 有突变 7 3(42.9) 无突变 29 19(65.6) IFN-γ通路 1.000 有突变 2 1(50.0) 无突变 34 21(61.8) 组蛋白乙酰化 0.511 有突变 2 2(100.0) 无突变 34 20(58.8) DNA甲基化 0.511 有突变 2 2(100.0) 无突变 34 20(58.8) 染色质重组 0.511 有突变 2 2(100.0) 无突变 34 20(58.8) 表 5 BR治疗MZL患者的不良反应
n=48,例(%) 不良反应 1~2级 3~4级 血液学不良反应 中性粒细胞减少 12(25.0) 6(12.5) 血小板减少 8(16.7) 2(4.2) 贫血 7(14.6) 1(2.1) 感染 粒细胞缺乏伴发热 1(2.1) 0 肺部感染 1(2.1) 1(2.1) 其他 恶心 14(29.2) 0 呕吐 7(14.6) 0 皮疹 8(16.7) 0 输液反应 2(4.2) 0 -
[1] 中国临床肿瘤学会指南工作委员会. 中国临床肿瘤学会(CSCO)淋巴瘤诊疗指南(2021年版)[M]. 北京: 人民卫生出版社, 2021: 233-251.
[2] Non-Hodgkin's Lymphoma Classifification Project. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project[J]. Blood, 1997, 89(11): 3909-3918. doi: 10.1182/blood.V89.11.3909
[3] 赵夏, 王黎, 张晟婷, 等. 90例原发胃肠道黏膜相关淋巴组织结外边缘区B细胞淋巴瘤患者临床特征与预后分析[J]. 中华血液学杂志, 2015, 36(1): 24-28.
[4] 闫姣, 何翠颖, 王连静, 等. 结外边缘区淋巴瘤患者遗传学改变的研究进展[J]. 国际输血及血液学杂志, 2021, 44(4): 356-361. doi: 10.3760/cma.j.cn511693-20201222-00260
[5] 王亚文, 徐佳岱, 阿孜古丽·麦合麦提, 等. 苯达莫司汀联合利妥昔单抗治疗初治边缘区B细胞淋巴瘤的疗效和安全性研究: 一项基于倾向性评分匹配的多中心回顾性研究[J]. 临床血液学杂志, 2022, 35(1): 35-40. http://www.whuhzzs.com/article/doi/10.13201/j.issn.1004-2806.2022.01.007
[6] Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial[J]. Lancet, 2013, 381(9873): 1203-1210. doi: 10.1016/S0140-6736(12)61763-2
[7] Kiesewetter B, Mayerhoefer ME, Lukas J, et al. Rituximab plus bendamustine is active in pretreated patients with extragastric marginal zone B cell lymphoma of the mucosa-associated lymphoid tissue(MALT lymphoma)[J]. Ann Hematol, 2014, 93(2): 249-253. doi: 10.1007/s00277-013-1865-3
[8] Carrillo-Cruz E, Marín-Oyaga VA, de la Cruz Vicente F, et al. Role of 18F-FDG-PET/CT in the management of marginal zone B cell lymphoma[J]. Hematol Oncol, 2015, 33(4): 151-158. doi: 10.1002/hon.2181
[9] Jaramillo Oquendo C, Parker H, Oscier D, et al. Systematic review of somatic mutations in Splenic Marginal Zone Lymphoma[J]. Sci Rep, 2019, 9(1): 10444. doi: 10.1038/s41598-019-46906-1
[10] Parry M, Rose-Zerilli MJ, Ljungström V, et al. Genetics and Prognostication in Splenic Marginal Zone Lymphoma: Revelations from Deep Sequencing[J]. Clin Cancer Res, 2015, 21(18): 4174-4183. doi: 10.1158/1078-0432.CCR-14-2759
[11] Savitsky K, Bar-Shira A, Gilad S, et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase[J]. Science, 1995, 268(5218): 1749-1753. doi: 10.1126/science.7792600
[12] Choi M, Kipps T, Kurzrock R. ATM Mutations in Cancer: Therapeutic Implications[J]. Mol Cancer Ther, 2016, 15(8): 1781-1791. doi: 10.1158/1535-7163.MCT-15-0945
[13] Stankovic T, Skowronska A. The role of ATM mutations and 11q deletions in disease progression in chronic lymphocytic leukemia[J]. Leuk Lymphoma, 2014, 55(6): 1227-1239. doi: 10.3109/10428194.2013.829919
[14] Hill HA, Qi X, Jain P, et al. Genetic mutations and features of mantle cell lymphoma: a systematic review and meta-analysis[J]. Blood Adv, 2020, 4(13): 2927-2938. doi: 10.1182/bloodadvances.2019001350
[15] Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms[J]. Blood, 2016, 127(20): 2375-2390. doi: 10.1182/blood-2016-01-643569
[16] Morin RD, Mendez-Lago M, Mungall AJ, et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma[J]. Nature, 2011, 476(7360): 298-303. doi: 10.1038/nature10351
[17] Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification[J]. J Clin Oncol, 2014, 32(27): 3059-3068. doi: 10.1200/JCO.2013.54.8800
[18] Sindel A, Al-Juhaishi T, Yazbeck V. Marginal Zone Lymphoma: State-of-the-Art Treatment[J]. Curr Treat Options Oncol, 2019, 20(12): 90. doi: 10.1007/s11864-019-0687-5
[19] Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study[J]. Blood, 2014, 123(19): 2944-2952.
[20] Flinn IW, van der Jagt R, Kahl B, et al. First-Line Treatment of Patients With Indolent Non-Hodgkin Lymphoma or Mantle-Cell Lymphoma With Bendamustine Plus Rituximab Versus R-CHOP or R-CVP: Results of the BRIGHT 5-Year Follow-Up Study[J]. J Clin Oncol, 2019, 37(12): 984-991. doi: 10.1200/JCO.18.00605
[21] Murakami H, Yoshioka T, Moriyama T, et al. Bendamustine Plus Rituximab as Salvage Treatment for Patients with Relapsed or Refractory Low-grade B-cell Lymphoma and Mantle Cell Lymphoma: A Single-Center Retrospective Study[J]. Acta Med Okayama, 2021, 75(4): 461-469. https://link.springer.com/article/10.1007/s00277-014-2077-1
[22] Karadurmus N, Paydas S, Esin E, et al. Effectiveness of bendamustine in relapsed or refractory lymphomacases: a Turkish Oncology Group study[J]. Arch Med Sci, 2019, 17(4): 920-927.
[23] Iannitto E, Bellei M, Amorim S, et al. Efficacy of bendamustine and rituximab in splenic marginal zone lymphoma: results from the phase Ⅱ BRISMA/IELSG36 study[J]. Br J Haematol, 2018, 183(5): 755-765. doi: 10.1111/bjh.15641
[24] Kiesewetter B, Raderer M. How can we assess and measure prognosis for MALT lymphoma? A review of current findings and strategies[J]. Expert Rev Hematol, 2021, 14(4): 391-399. doi: 10.1080/17474086.2021.1909468
[25] 王芳, 韩雪, 白贝贝, 等. 伴单克隆免疫球蛋白边缘带淋巴瘤三例报告及文献复习[J]. 中华血液学杂志, 2016, 37(1): 39-44.
[26] Salar A, Domingo-Domenech E, Panizo C, et al. Long-term results of a phase 2 study of rituximab and bendamustine for mucosa-associated lymphoid tissue lymphoma[J]. Blood, 2017, 130(15): 1772-1774.
[27] Alderuccio JP, Arcaini L, Watkins MP, et al. An international analysis evaluating frontline bendamustine with rituximab in extranodal marginal zone lymphoma[J]. Blood Adv, 2022, 6(7): 2035-2044.
[28] Laribi K, Tempescul A, Ghnaya H, et al. The bendamustine plus rituximab regimen is active against primary nodal marginal zone B-cell lymphoma[J]. Hematol Oncol, 2017, 35(4): 536-541. https://onlinelibrary.wiley.com/doi/abs/10.1002/hon.2334
[29] Arcaini L, Rossi D, Paulli M. Splenic marginal zone lymphoma: from genetics to management[J]. Blood, 2016, 127(17): 2072-2081. https://moh-it.pure.elsevier.com/en/publications/splenic-marginal-zone-lymphoma-from-genetics-to-management
[30] Zhang GP, Cao PF, Feng LJ. Detection and clinical significance of genes in primary gastrointestinal MALT lymphoma[J]. Tumour Biol, 2014, 35(4): 3223-3228. https://link.springer.com/article/10.1007/s13277-013-1421-8
[31] Salido M, Baró C, Oscier D, et al. Cytogenetic aberrations and their prognostic value in a series of 330 splenic marginal zone B-cell lymphomas: a multicenter study of the Splenic B-Cell Lymphoma Group[J]. Blood, 2010, 116(9): 1479-1488. http://public-files.prbb.org/publicacions/29613d80-faec-012d-a845-000c293b26d5.pdf
[32] Salar A, Domingo-Domenech E, Panizo C, et al. First-line response-adapted treatment with the combination of bendamustine and rituximab in patients with mucosa-associated lymphoid tissue lymphoma(MALT2008-01): a multicentre, single-arm, phase 2 trial[J]. Lancet Haematol, 2014, 1(3): e104-111. https://www.sciencedirect.com/science/article/pii/S2352302614000210
-




 下载:
下载: