CAR-T related hemophagocytic lymphohistiocytosis in multiple myeloma: A case report and literature review
-
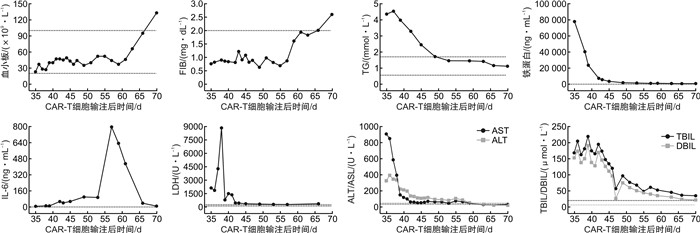
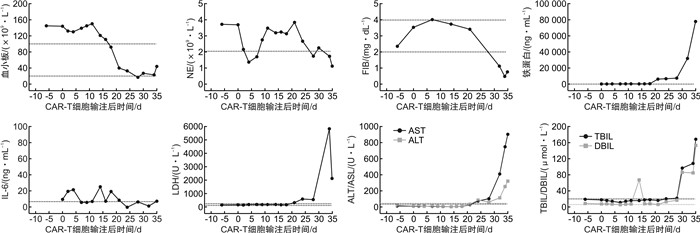
摘要: 目的探讨嵌合抗原受体T细胞(CAR-T)治疗合并噬血细胞综合征(HLH)的发病机制、临床特征及诊疗。方法回顾性分析1例复发难治性多发性骨髓瘤患者CAR-T治疗发生HLH的临床表现、诊疗经过及后期随访, 并结合相关文献报道, 分析CAR-T治疗的相关毒副反应及诊疗方案。结果该例患者CAR-T输注后第2天即发生1级细胞因子释放综合征(CRS), 输注后第35天出现3级CRS, 且合并HLH, 给予甲泼尼龙及托珠单抗治疗后缓解, 现随访15个月余, 原发病维持严格意义的完全缓解。结论CAR-T相关HLH发生率虽然极低, 但临床医师应该保持警惕, 并延长观察时间, 其治疗方案可参考HLH-2004方案Abstract: ObjectiveTo investigate the pathogenesis, clinical features, diagnosis and treatment of hemophagocytic lymphohistiocytosis(HLH) associated with chimeric antigen receptor T cell(CAR-T) therapy.MethodsThe clinical manifestations, diagnosis, treatment and follow-up of a relapsed and refractory multiple myeloma patient who underwent CAR-T associated HLH were retrospectively analyzed, and the related toxic and adverse events of CAR-T therapy and treatment plan were analyzed in combination with relevant literature.ResultsCytokine release syndrome(CRS) occurred in the patient +2 d after CAR-T infusion, and grade 3 CRS occurred +35 d later with HLH, which was relieved after methylprednisolone and tozizumab treatment. The patient is now followed up for more than 15 months, and the primary disease remains stringent complete response.ConclusionAlthough the incidence of CAR-T associated HLH is extremely low, clinicians should remain vigilant and extend the observation period, and treatment options can be referred to HLH-2004.
-

-
[1] Muhammad N, Mao Q, Xia H. CAR T-cells for cancer therapy[J]. Biotechnol Genet Eng Rev, 2017, 33(2): 190-226. doi: 10.1080/02648725.2018.1430465
[2] Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial[J]. Lancet, 2015, 385(9967): 517-528. https://www.sciencedirect.com/science/article/pii/S0140673614614033
[3] Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia[J]. N Engl J Med, 2018, 378(5): 439-448. doi: 10.1056/NEJMoa1709866
[4] Kochenderfer JN, Rosenberg SA. Chimeric antigen receptor-modified T cells in CLL[J]. N Engl J Med, 2011, 365(20): 1937-1938;author reply 1938. doi: 10.1056/NEJMc1111004
[5] Turtle CJ, Hay KA, Hanafi LA, et al. Durable Molecular Remissions in Chronic Lymphocytic Leukemia Treated With CD19-Specific Chimeric Antigen Receptor-Modified T Cells After Failure of Ibrutinib[J]. J Clin Oncol, 2017, 35(26): 3010-3020. doi: 10.1200/JCO.2017.72.8519
[6] Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor[J]. J Clin Oncol, 2015, 33(6): 540-549. doi: 10.1200/JCO.2014.56.2025
[7] Kochenderfer JN, Somerville R, Lu T, et al. Lymphoma Remissions Caused by Anti-CD19 Chimeric Antigen Receptor T Cells Are Associated With High Serum Interleukin-15 Levels[J]. J Clin Oncol, 2017, 35(16): 1803-1813. doi: 10.1200/JCO.2016.71.3024
[8] Mikkilineni L, Kochenderfer JN. CAR T cell therapies for patients with multiple myeloma[J]. Nat Rev Clin Oncol, 2021, 18(2): 71-84. doi: 10.1038/s41571-020-0427-6
[9] Yan Z, Cao J, Cheng H, et al. A combination of humanised anti-CD19 and anti-BCMA CAR T cells in patients with relapsed or refractory multiple myeloma: a single-arm, phase 2 trial[J]. Lancet Haematol, 2019, 6(10): e521-e529. doi: 10.1016/S2352-3026(19)30115-2
[10] Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome[J]. Blood, 2014, 124(2): 188-195. doi: 10.1182/blood-2014-05-552729
[11] Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management[J]. Blood, 2016, 127(26): 3321-3330.
[12] Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma[J]. N Engl J Med, 2017, 377(26): 2531-2544. doi: 10.1056/NEJMoa1707447
[13] Jordan MB, Allen CE, Greenberg J, et al. Challenges in the diagnosis of hemophagocytic lymphohistiocytosis: Recommendations from the North American Consortium for Histiocytosis(NACHO)[J]. Pediatr Blood Cancer, 2019, 66(11): e27929. https://pubmed.ncbi.nlm.nih.gov/31339233/
[14] 刘艺, 郭涛. 血液系统肿瘤治疗相关噬血细胞综合征的诊疗进展[J]. 临床血液学杂志, 2022, 35(1): 16-20. http://www.whuhzzs.com/article/doi/10.13201/j.issn.1004-2806.2022.01.004
[15] Sandler RD, Tattersall RS, Schoemans H, et al. Diagnosis and Management of Secondary HLH/MAS Following HSCT and CAR-T Cell Therapy in Adults; A Review of the Literature and a Survey of Practice Within EBMT Centres on Behalf of the Autoimmune Diseases Working Party(ADWP)and Transplant Complications Working Party(TCWP)[J]. Front Immunol, 2020, 11: 524.
[16] Ishii K, Pouzolles M, Chien CD, et al. Perforin-deficient CAR T cells recapitulate late-onset inflammatory toxicities observed in patients[J]. J Clin Invest, 2020, 130(10): 5425-5443. doi: 10.1172/JCI130059
[17] Teachey DT, Lacey SF, Shaw PA, et al. Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-cell Therapy for Acute Lymphoblastic Leukemia[J]. Cancer Discov, 2016, 6(6): 664-679. doi: 10.1158/2159-8290.CD-16-0040
[18] Teachey DT, Rheingold SR, Maude SL, et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy[J]. Blood, 2013, 121(26): 5154-5157. https://www.semanticscholar.org/paper/Cytokine-release-syndrome-after-blinatumomab-to-and-Teachey-Rheingold/a7b8309d2b92d216d5738272e243ce2a8d29eb7d
[19] Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy-assessment and management of toxicities[J]. Nat Rev Clin Oncol, 2018, 15(1): 47-62. https://www.nature.com/articles/nrclinonc.2017.148
[20] Henter JI, Horne A, Aricó M, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis[J]. Pediatr Blood Cancer, 2007, 48(2): 124-131. https://onlinelibrary.wiley.com/doi/abs/10.1002/pbc.21039
[21] Canna SW, Marsh RA. Pediatric hemophagocytic lymphohistiocytosis[J]. Blood, 2020, 135(16): 1332-1343.
[22] Dholaria BR, Bachmeier CA, Locke F. Mechanisms and Management of Chimeric Antigen Receptor T-Cell Therapy-Related Toxicities[J]. BioDrugs, 2019, 33(1): 45-60. https://link.springer.com/article/10.1007/s40259-018-0324-z
[23] Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia[J]. N Engl J Med, 2014, 371(16): 1507-1517. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4267531/
-




 下载:
下载: