The value of venetoclax blood concentration monitoring in the treatment of acute myeloid leukemia and the efficacy and safety of combined azacytidine in the treatment of acute myeloid leukemia
-
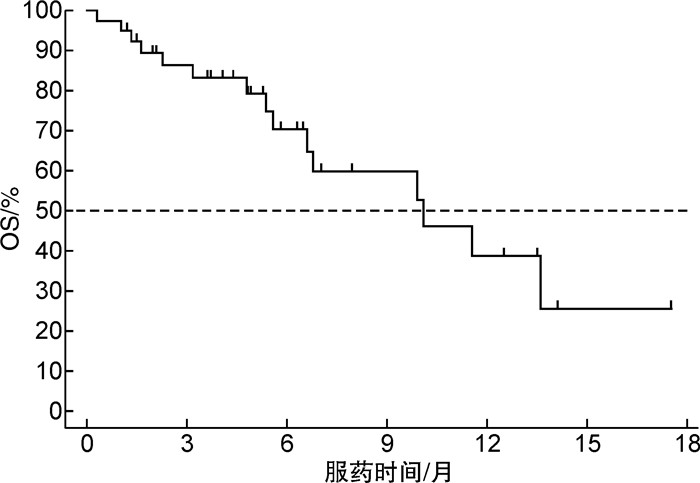
摘要: 目的评价维奈克拉血药浓度对治疗急性髓系白血病(AML)疗效及安全性的影响,并分析维奈克拉联合阿扎胞苷治疗AML的疗效与安全性。方法回顾性分析2020年7月—2021年12月我院接受维奈克拉联合治疗的40例AML患者的临床资料,维奈克拉血药浓度检测采用HPLC-MS/MS法。结果随访2.5~17.5个月,中位随访6.1个月。总缓解率为70.0%(28/40),其中16例(40.0%)获得完全缓解,8例(20.0%)获得骨髓完全缓解伴不完全血液学恢复,4例(10.0%)获得部分缓解。发生Ⅲ~Ⅳ级中性粒细胞减少、血小板计数下降的患者分别为23例(57.5%)和22例(55.0%),1例患者因Ⅲ~Ⅳ级血液学毒性和感染而停药,无临床肿瘤溶解综合征发生。40例患者的中位维奈克拉血药浓度为1.6(0.2~7.8)μg/mL,血药浓度 < 1.0 μg/mL、1.0~3.2 μg/mL、>3.2 μg/mL组的总有效率分别为36.4%(4/11)、89.5%(17/19)、70.0%(7/10),≥Ⅲ级中性粒细胞减少的发生率分别为36.4%(4/11)、47.4%(9/19)、100.0%(10/10),≥Ⅲ级血小板计数降低的发生率分别为45.5%(5/11)、42.1%(8/19)、90.0%(9/10)。维奈克拉血药浓度与中性粒细胞计数(R2=0.485)及血小板计数(R2=0.457)存在一定的线性关系(均P < 0.05)。40例患者中15例(37.5%)死亡,中位总生存期为10.1(0.3~17.5)个月。结论维奈克拉联合阿扎胞苷较维奈克拉单药治疗缓解率高,总生存时间长,且严重不良反应少。监测维奈克拉血药浓度可以提高用药安全性,减少不良反应的发生。Abstract: ObjectiveTo evaluate the efficacy and safety of venetoclax in the treatment of acute myeloid leukemia(AML), and to analyze the efficacy and safety of venetoclax combined with azacytidine in the treatment of AML.MethodsClinical data of 40 patients with AML who received venetoclax combination therapy in our hospital from July 2020 to December 2021 were retrospectively analyzed. Plasma concentration of venetoclax was determined by HPLC-MS/MS.ResultsThe median follow-up time was 6.1(2.5 to 17.5) months. The overall response rate was 70.0%(28/40), of which 16 cases(40.0%) achieved complete response, 8 cases(20.0%) achieved complete response with incomplete hematologic recovery, and 4 cases(10.0%) achieved partial response. There were 23 cases(57.5%) and 22 cases(55.0%) with grade Ⅲ-Ⅳ neutropenia and thrombocytopenia, respectively. One case stopped medication due to grade Ⅲ-Ⅳhematological toxicity and infection. There was no clinical tumor lysis syndrome. The median plasma concentration of venetoclax in 40 patients was 1.6(0.2-7.8) μg/mL. In groups of venetoclax concentration < 1.0 μg/mL, 1.0-3.2 μg/mL, >3.2 μg/mL, the overall response rate was 36.4%(4/11), 89.5%(17/19) and 70.0%(7/10), the incidence of grade ≥Ⅲ neutropenia was 36.4%(4/11), 47.4%(9/19) and 100.0%(10/10), and the incidence of grade ≥Ⅲ thrombocytopenia was 45.5%(5/11), 42.1%(8/19) and 90.0%(9/10), respectively. There was a certain linear relationship between the serum concentration of venetoclax and neutrophil count(R2=0.485, P < 0.05) and platelet count(R2=0.457, P < 0.05). Among the 40 patients, 15 cases(37.5%) died, and the median overall survival was 10.1(0.3-17.5) months.ConclusionCompared with venetoclax monotherapy, venetoclax combined with azacytidine has a higher remission rate, longer overall survival time, and less serious adverse events. Monitoring the blood concentration of venetoclax can improve drug safety and reduce adverse events.
-
Key words:
- acute myeloid leukemia /
- venetoclax /
- azacitidine /
- curative effect /
- blood concentration
-

-
表 1 患者临床特征与CR的相关性分析
例 临床特征 获得CR
(16例)未获得CR
(24例)P 年龄 < 60岁 7 11 1.00 ≥60岁 9 13 性别 男 8 13 1.00 女 8 11 既往治疗次数 0 6 8 0.81 < 3次 5 8 ≥3次 5 8 维奈克拉总疗程 < 3个 11 21 0.29 ≥3个 5 3 移植情况 否 15 24 0.83 是 1 0 预后情况 预后良好 7 3 0.79 预后中等 1 7 预后不良 8 14 表 2 维奈克拉最高血药浓度不同分组的临床疗效及不良反应比较
维奈克拉血药浓度 例数 ORR/例(%) 不良反应/例(%) ≥Ⅲ级中性粒细胞减少 ≥Ⅲ级血小板计数降低 < 1.0 μg/mL 11 4(36.4) 4(36.4) 5(45.5) 1.0~3.2 μg/mL 19 17(89.5) 9(47.4) 8(42.1) >3.2 μg/mL 10 7(70.0) 10(100.0) 9(90.0) -
[1] Estey E, Karp JE, Emadi A, et al. Recent drug approvals for newly diagnosed acute myeloid leukemia: gifts or a Trojan horse?[J]. Leukemia, 2020, 34(3): 671-681.
[2] 中华医学会血液学分会白血病淋巴瘤学组. 中国成人急性髓系白血病(非急性早幼粒细胞白血病)诊疗指南(2021年版)[J]. 中华血液学杂志, 2021, 42(8): 617-623.
[3] Leonard JP, Martin P, Roboz GJ. Practical Implications of the 2016 Revision of the World Health Organization Classification of Lymphoid and Myeloid Neoplasms and Acute Leukemia[J]. J Clin Oncol, 2017, 35(23): 2708-2715. doi: 10.1200/JCO.2017.72.6745
[4] 王磊, 代玉超, 李梦, 等. 测定血液病患者血浆Venetoclax浓度的HPLC-MS/MS方法[J]. 分子诊断与治疗杂志, 2021, 13(3): 413-417. https://www.cnki.com.cn/Article/CJFDTOTAL-YXYQ202103020.htm
[5] Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019[J]. CA Cancer J Clin, 2019, 69(1): 7-34. doi: 10.3322/caac.21551
[6] Ali S, Dunmore HM, Karres D, et al. The EMA review of mylotarg(gemtuzumab ozogamicin)for the treatment of acute myeloid leukemia[J]. Oncologist, 2019, 24(5): e171-e179. doi: 10.1634/theoncologist.2019-0025
[7] Wei W, Zeng H, Zheng R, et al. Cancer registration in China and its role in cancer prevention and control[J]. Lancet Oncol, 2020, 21(7): e342-e349. doi: 10.1016/S1470-2045(20)30073-5
[8] King AC, Peterson TJ, Horvat TZ, et al. Venetoclax: A First-in-Class Oral BCL-2 Inhibitor for the Management of Lymphoid Malignancies[J]. Ann Pharmacother, 2017, 51(5): 410-416. doi: 10.1177/1060028016685803
[9] Maiti A, DiNardo CD, Qiao W, et al. Ten-day decitabine with venetoclax versus intensive chemotherapy in relapsed or refractory acute myeloid leukemia: A propensity score-matched analysis[J]. Cancer, 2021, 127(22): 4213-4220. doi: 10.1002/cncr.33814
[10] DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia[J]. N Engl J Med, 2020, 383(7): 617-629. doi: 10.1056/NEJMoa2012971
[11] Winters AC, Gutman JA, Purev E, et al. Real-world experience of venetoclax with azacitidine for untreated patients with acute myeloid leukemia[J]. Blood Adv, 2019, 3(20): 2911-2919. doi: 10.1182/bloodadvances.2019000243
[12] Yang Y, Fu LJ, Chen CM, et al. Venetoclax in combination with chidamide and dexamethasone in relapsed/refractory primary plasma cell leukemia without t(11;14): A case report[J]. World J Clin Cases, 2021, 9(5): 1175-1183. doi: 10.12998/wjcc.v9.i5.1175
[13] Bewersdorf JP, Giri S, Wang R, et al. Venetoclax as monotherapy and in combination with hypomethylating agents or low dose cytarabine in relapsed and treatment refractory acute myeloid leukemia: a systematic review and meta-analysis[J]. Haematologica, 2020, 105(11): 2659-2663. doi: 10.3324/haematol.2019.242826
[14] Wei AH, Strickland SA Jr, Hou JZ, et al. Venetoclax Combined With Low-Dose Cytarabine for Previously Untreated Patients With Acute Myeloid Leukemia: Results From a Phase Ib/Ⅱ Study[J]. J Clin Oncol, 2019, 37(15): 1277-1284. doi: 10.1200/JCO.18.01600
[15] 盘婉盈, 张映璇, 涂三芳, 等. Venetoclax联合去甲基化药物治疗复发/难治性急性髓系白血病的疗效及预后分析[J]. 临床血液学杂志, 2021, 34(9): 650-654. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXZ202109010.htm
[16] 战榕. Bcl-2抑制剂在急性髓系白血病中的研究进展: 第62届美国血液学年会报道[J]. 临床血液学杂志, 2021, 34(5): 298-301. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXZ202105002.htm
[17] 黄凯凯, 吴微, 朱华民, 等. BCL-2抑制剂Venetoclax联合阿扎胞苷治疗难治复发急性髓系白血病1例[J]. 现代肿瘤医学, 2022, 30(1): 132-134. https://www.cnki.com.cn/Article/CJFDTOTAL-SXZL202201029.htm
[18] 赵爽, 赵宏伟, 马威. 维奈托克对急性髓系白血病肿瘤标志基因和炎症因子的影响[J]. 医药导报, 2018, 37(1): 31-34. https://www.cnki.com.cn/Article/CJFDTOTAL-YYDB201801008.htm
[19] Salem AH, Dunbar M, Agarwal SK. Pharmacokinetics of venetoclax in patients with 17p deletion chronic lymphocytic leukemia[J]. Anticancer Drugs, 2017, 28(8): 911-914.
[20] Agarwal SK, DiNardo CD, Potluri J, et al. Management of Venetoclax-Posaconazole Interaction in Acute Myeloid Leukemia Patients: Evaluation of Dose Adjustments[J]. Clin Ther, 2017, 39(2): 359-367. https://www.sciencedirect.com/science/article/pii/S014929181730036X
[21] Cheng FM, Tien JZ, Chen TT, et al. Venetoclax plus cytochrome P450 inhibitors without ramp-up strategy led to low risk of tumor lysis syndrome in acute myeloid leukemia[J]. Ann Hematol, 2020, 99(9): 2193-2195.
[22] Salem AH, Dave N, Marbury T, et al. Pharmacokinetics of the BCL-2 Inhibitor Venetoclax in Subjects with Hepatic Impairment[J]. Clin Pharmacokinet, 2019, 58(8): 1091-1100. https://accp1.onlinelibrary.wiley.com/doi/10.1002/cpdd.395
[23] DiNardo CD, Pratz KW, Letai A, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study[J]. Lancet Oncol, 2018, 19(2): 216-228. https://www.sciencedirect.com/science/article/pii/S147020451830010X
[24] Schuler E, Wagner-Drouet EM, Ajib S, et al. Treatment of myeloid malignancies relapsing after allogeneic hematopoietic stem cell transplantation with venetoclax and hypomethylating agents-a retrospective multicenter analysis on behalf of the German Cooperative Transplant Study Group[J]. Ann Hematol, 2021, 100(4): 959-968.
[25] Cheson BD, Heitner Enschede S, Cerri E, et al. Tumor Lysis Syndrome in Chronic Lymphocytic Leukemia with Novel Targeted Agents[J]. Oncologist, 2017, 22(11): 1283-1291.
-




 下载:
下载: