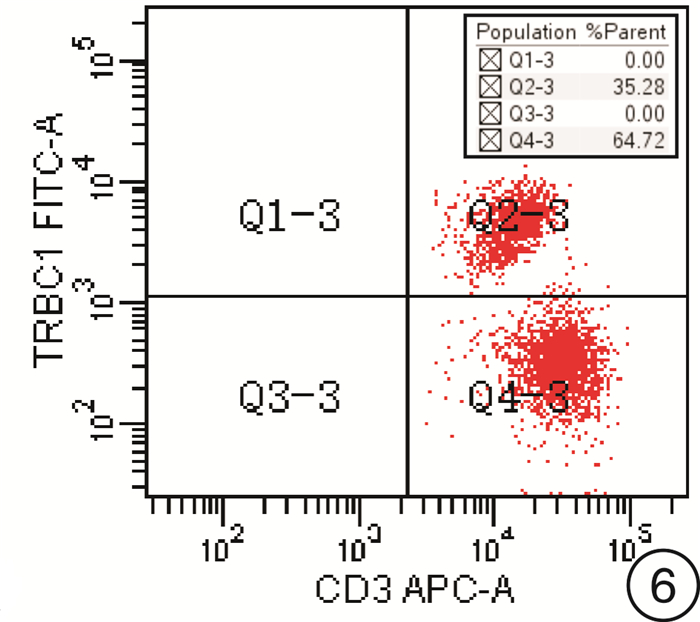
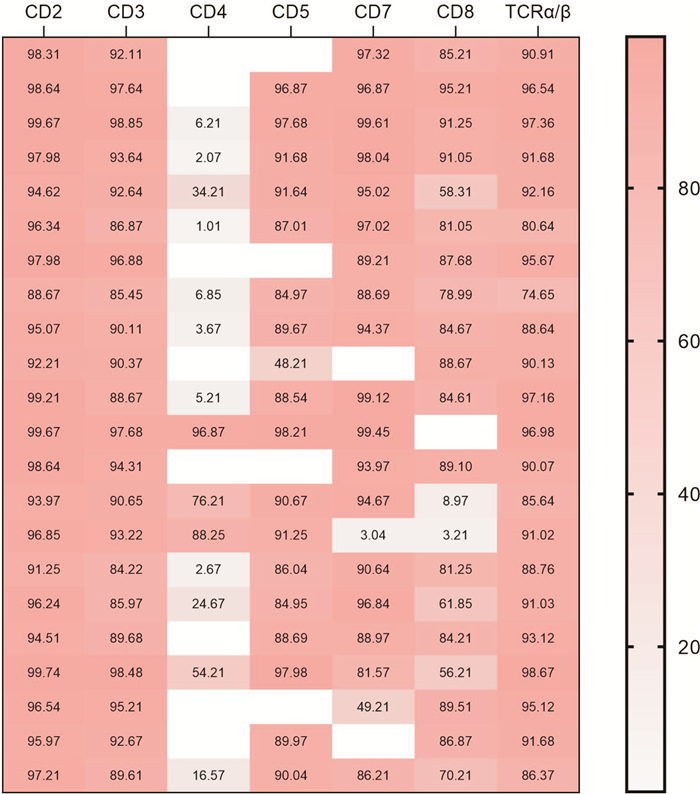
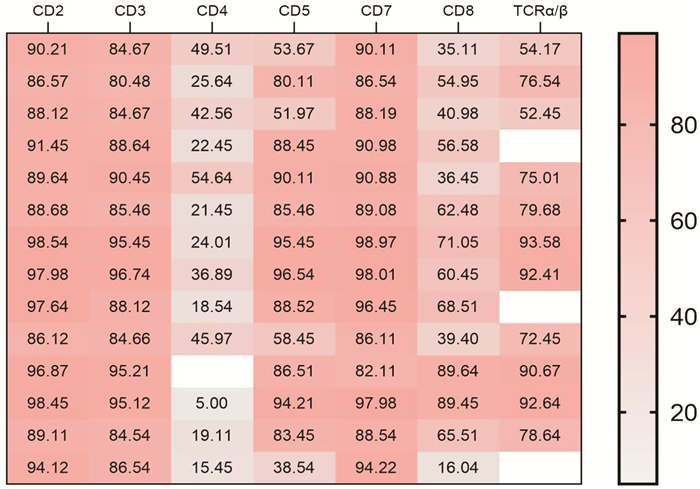
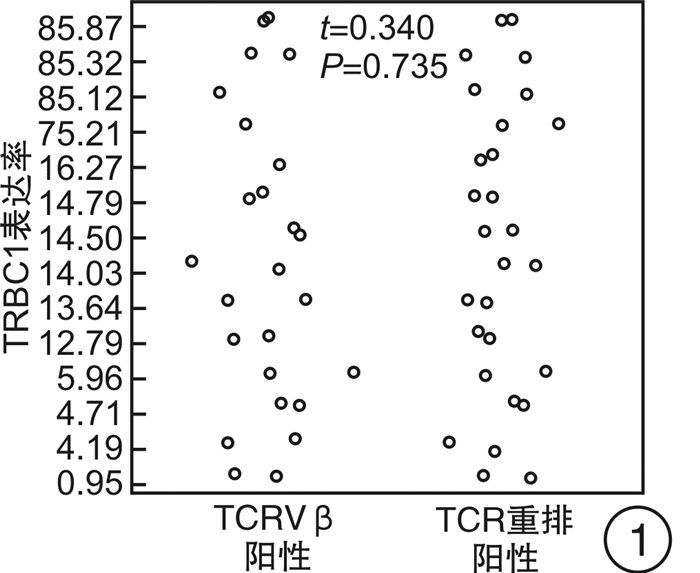
-
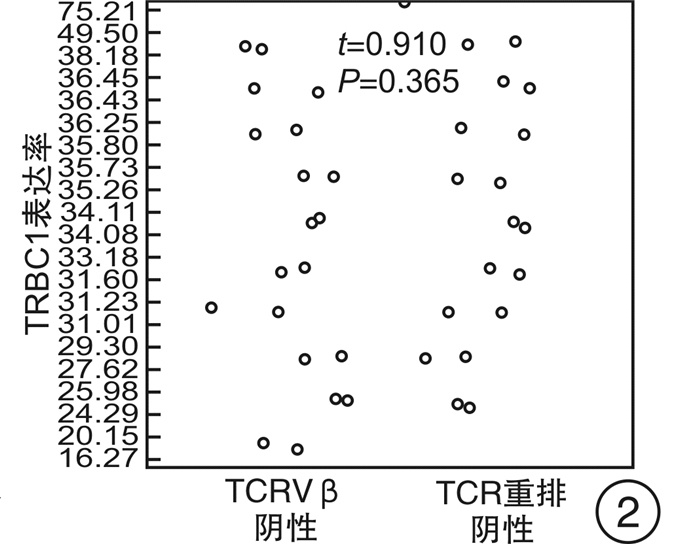
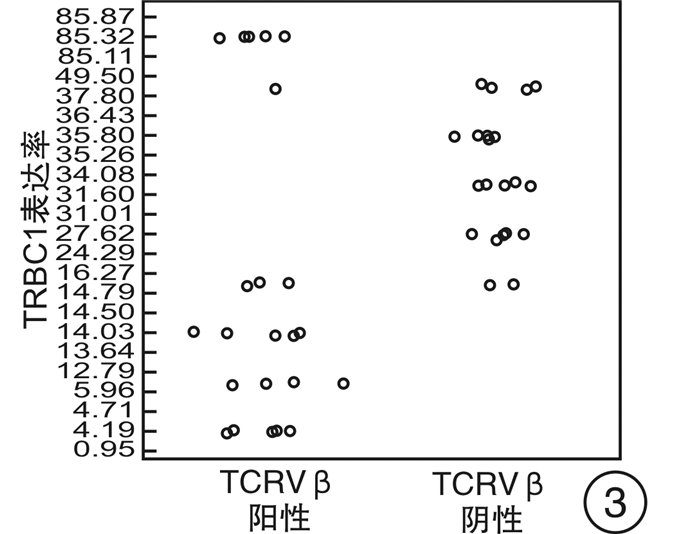
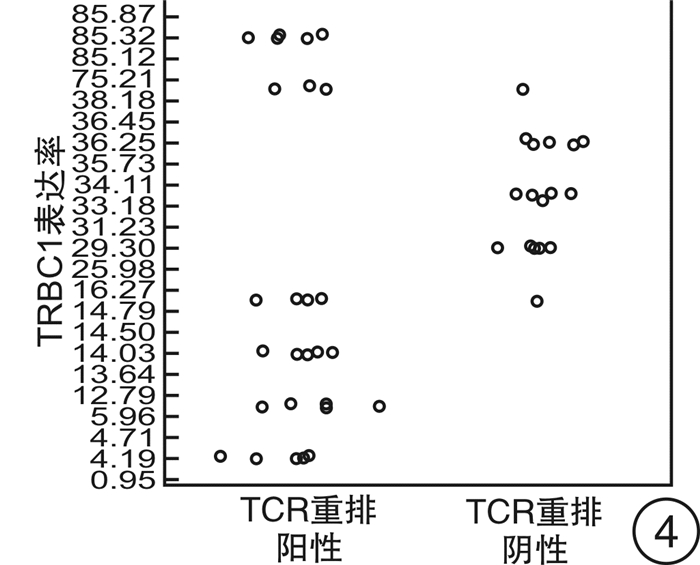
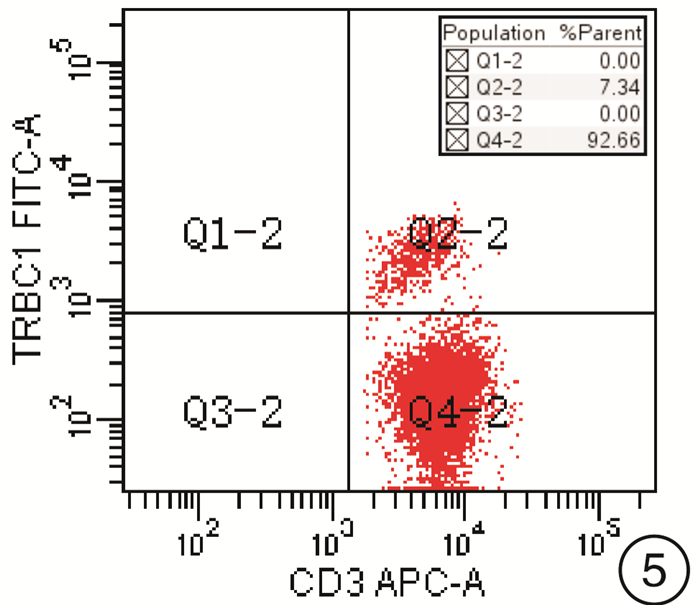
摘要: 目的 研究使用流式细胞术T细胞抗原受体β恒定区1(TRBC1)基因单克隆抗体鉴别T细胞克隆方法的可靠性。方法 回顾2021年6月—2022年2月的78例样本,分别使用流式细胞术TRBC1标记法、T细胞抗原受体可变区β链(TCRVβ)法及分子T细胞抗原受体(TCR)重排法,通过几种方法比较得到TRBC1判断T细胞克隆的可行性。并比较TRBC1法在不同疾病中的表达及与其他相关CD分子的共表达情况是否存在差异。结果 使用TRBC1法的表达率 < 15%和>85%作为克隆阳性判断标准,与TCRVβ法及TCR重排法判断克隆性比较,差异无统计学意义(P=1.000,0.250);且其kappa值分别为0.871和0.873,为高度吻合性。在不同疾病T细胞克隆判断中,除TCRγ/δ淋巴瘤外,在其他疾病T细胞克隆判断上均差异无统计学意义(P>0.05)。TRBC1的表达率与CD2、CD3、CD4、CD5、CD7、CD8、TCRα/β表达均无相关性(|r| < 0.3),可作为独立的判断因素。结论 使用TRBC1法同其他方法比较具有较高的一致性,方法可靠;且其经济高效,试验方法简单,值得临床作为T系克隆的判断方法进行推广。Abstract: Objective To investigate the reliability of flow cytometry TRBC1 monoclonal antibody in identifying T cell clones.Methods Reviewing seventy-eight samples from June 2021 to February 2022, TRBC1 method, TCRVβ method and TCR rearrangement method were respectively used to compare the feasibility of TRBC1 to identify T cell clones. The expression of TRBC1 with different CD molecules and in different diseases was compared.Results When TRBC1 expression rate in < 15% and >85% was used as the criterion for determining clonal positive, compared with TCRVβ method and TCR rearrangement method, the P values were 1.000 and 0.250 respectively, and there was no significant difference. The kappa values were 0.871 and 0.873, respectively, indicating high consistency. There was no significant difference in the clonal judgment of T cells in different diseases except TCRγ/δ lymphoma(P>0.05). CD2, CD3, CD4, CD5, CD7, CD8, TCRα/β expression and TRBC1 expression had no correlation(|r| < 0.3), which could be used as an independent diagnostic factor.Conclusion Compared with other methods, TRBC1 method may hanve a high consistency. It may be economical, efficient and simple. It is worthy of clinical application as a judgment method of T-line cloning in clinical practice.
-
Key words:
- T cell clones /
- flow cytometry /
- TRBC1 /
- monoclonal antibody
-

-
表 1 样本的基本信息
例 性别 >50岁 ≤50岁 合计 男 23 15 38 女 25 15 40 合计 48 30 78 表 2 TRBC1的2种判断标准比较
例 方法 表达率判断法 阳性 阴性 合计 比例判断法 阳性 40 18 58 阴性 2 18 20 合计 42 36 78 表 3 TCRVβ法与TRBC1 2种判断方法的比较
例 方法 比例判断法 表达率判断法 阳性 阴性 合计 阳性 阴性 合计 TCRVβ法 阳性 37 4 41 39 2 41 阴性 21 16 37 3 34 37 合计 58 20 78 42 36 78 表 4 TCR重排法与TRBC1 2种判断方法比较
例 方法 比例判断法 表达率判断法 阳性 阴性 合计 阳性 阴性 合计 TCR重排法 阳性 24 5 29 26 3 29 阴性 11 8 19 0 19 19 合计 35 13 48 26 22 48 表 5 使用TRBC1法、TCRVβ法及TCR重排法比较
例 方法 阳性 阴性 合计 TRBC1法 26 22 48 TCRVβ法 26 22 48 TCR重排法 29 19 48 表 6 不同疾病使用3种方法一致性比较
疾病名称 例数 TCRVβ检测 TCR重排 TRBC1阳性 结论 γ/δ T淋巴瘤 5 阴性 阳性 阴性 不一致 T淋巴细胞增殖1) 22 阴性 阴性(8例无TCR重排) 阴性 一致 T细胞幼淋细胞白血病 2 阳性 无 阳性 一致 T细胞幼淋细胞白血病 1 阴性 阳性 阳性 不一致 成人T细胞白血病/淋巴瘤 6 阳性 阳性(2例无TCR重排) 阳性 一致 外周T细胞淋巴瘤 8 阳性 阳性(3例无TCR重排) 阳性 一致 血管母T细胞淋巴瘤 7 阳性 阳性(3例无TCR重排) 阳性 一致 1)T淋巴细胞占淋巴细胞比例增高。 表 7 T大颗粒细胞淋巴瘤使用TRBC1法、TCRVβ法及TCR重排法比较
例 方法 阳性 阴性 合计 TRBC1法 13 2 15 TCRVβ法 13 2 15 TCR重排法 15 0 15 合计 41 4 45 表 8 TRBC1的表达率与各CD分子的表达率相关性
类型 CD2 CD3 CD4 CD5 CD7 CD8 TCRα/β Pearson系数(r) -0.093 -0.164 -0.081 -0.012 -0.145 -0.127 0.055 |r| 0.093 0.164 0.081 0.012 0.145 0.127 0.055 -
[1] Ni X, Maiti S, Redko A, et al. Monitoring malignant T-cell clones by direct TCR expression assay in patients with leukemic cutaneous T-cell lymphoma during extracorporeal photopheresis[J]. Photodermatol Photoimmunol Photomed, 2022, 38(2): 158-168. doi: 10.1111/phpp.12732
[2] 商芳影, 郑金娥, 马耀坤, 等. 运用CLLflow积分系统诊断中国B细胞慢性淋巴细胞增殖性疾病的研究[J]. 临床血液学杂志, 2021, 34(12): 841-846. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXZ202112003.htm
[3] Maciocia PM, Wawrzyniecka PA, Philip B, et al. Targeting the T cell receptor β-chain constant region for immunotherapy of T cell malignancies[J]. Nat Med, 2017, 23(12): 1416-1423. doi: 10.1038/nm.4444
[4] Chen M, Wang A, Liu S, et al. Analysis of the Expression of the TRBC1 in T lymphocyte tumors[J]. Indian J Hematol Blood Transfus, 2021, 37(2): 271-279. doi: 10.1007/s12288-020-01357-x
[5] Horna P, Otteson GE, Shi M, et al. Flow Cytometric Evaluation of Surface and Cytoplasmic TRBC1 Expression in the Differential Diagnosis of Immature T-Cell Proliferations[J]. Am J Clin Pathol, 2022, 157(1): 64-72. doi: 10.1093/ajcp/aqab098
[6] Shi M, Jevremovic D, Otteson GE, et al. Single Antibody Detection of T-Cell Receptor αβ Clonality by Flow Cytometry Rapidly Identifies Mature T-Cell Neoplasms and Monotypic Small CD8-Positive Subsets of Uncertain Significance[J]. Cytometry B Clin Cytom, 2020, 98(1): 99-107. doi: 10.1002/cyto.b.21782
[7] Novikov ND, Griffin GK, Dudley G, et al. Utility of a Simple and Robust Flow Cytometry Assay for Rapid Clonality Testing in Mature Peripheral T-Cell Lymphomas[J]. Am J Clin Pathol, 2019, 151(5): 494-503. doi: 10.1093/ajcp/aqy173
[8] 杨瑞, 陈灿, 李沂玮, 等. 32例T大颗粒淋巴细胞白血病的临床特征分析[J]. 临床血液学杂志, 2020, 33(7): 508-513. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXZ202007015.htm
[9] Horna P. Single-Antibody Detection of T-Cell Receptor Beta Chain Monotypia Resolves Uncertainties in the Identification and Quantitation of Sézary Cells By Routine Flow Cytometry: Towards Accurate and Unequivocal Blood Staging of Cutaneous T-Cell Lymphomas[J]. Blood, 2019, 134(Suppl1): 2848-2848.
[10] Berg H, Otteson GE, Corley H, et al. Flow cytometric evaluation of TRBC1 expression in tissue specimens and body fluids is a novel and specific method for assessment of T-cell clonality and diagnosis of T-cell neoplasms[J]. Cytometry B Clin Cytom, 2021, 100(3): 361-369. doi: 10.1002/cyto.b.21881
[11] Shi M, Olteanu H, Jevremovic D, et al. T-cell clones of uncertain significance are highly prevalent and show close resemblance to T-cell large granular lymphocytic leukemia. Implications for laboratory diagnostics[J]. Mod Pathol, 2020, 33(10): 2046-2057. doi: 10.1038/s41379-020-0568-2
[12] Horna P, Shi M, Olteanu H, et al. Emerging Role of T-cell Receptor Constant β Chain-1(TRBC1) Expression in the Flow Cytometric Diagnosis of T-cell Malignancies[J]. Int J Mol Sci, 2021, 22(4): 1817. doi: 10.3390/ijms22041817
-

| 引用本文: | 马耀坤, 郑金娥, 李小青, 等. 应用TRBC1鉴别T细胞克隆的研究[J]. 临床血液学杂志, 2023, 36(4): 260-264. doi: 10.13201/j.issn.1004-2806.2023.04.008 |
| Citation: | MA Yaokun, ZHENG Jine, LI Xiaoqing, et al. Identification of T cell clones by TRBC1[J]. J Clin Hematol, 2023, 36(4): 260-264. doi: 10.13201/j.issn.1004-2806.2023.04.008 |
- Figure 1.
- Figure 2.
- Figure 3.
- Figure 4.
- Figure 5.
- Figure 6.
- Figure 7.
- Figure 8.



 下载:
下载: