Study of correlation between driver gene mutations and cytokines in Ph-negative myeloproliferative neoplasms
-
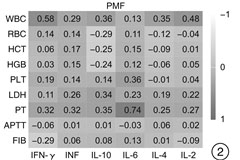
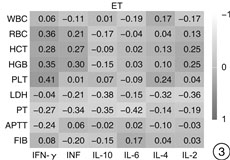
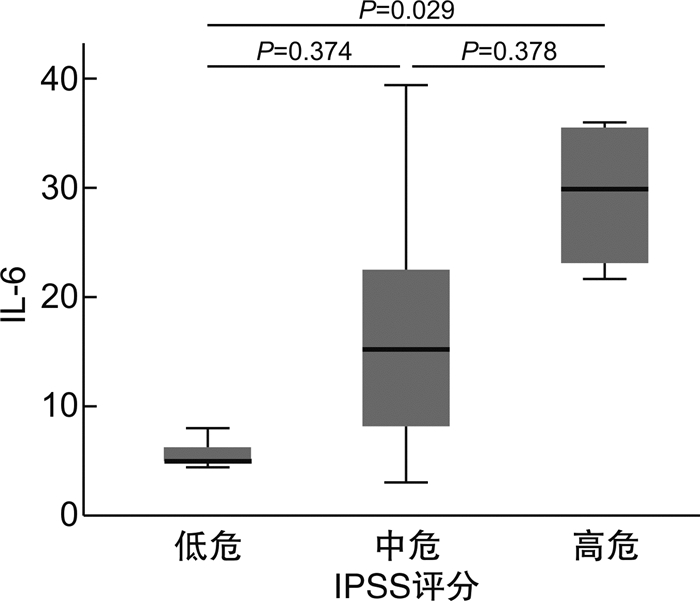
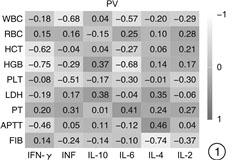
摘要: 目的 探究真实世界中经典骨髓增殖性肿瘤(myeloproliferative neoplasms,MPN)患者不同亚型及驱动突变状态的血清细胞因子[肿瘤坏死因子(TNF)、干扰素-γ(IFN-γ)、白细胞介素(IL)-10、IL-6、IL-4、IL-2]表达谱及细胞因子在疾病演化进展过程中的作用。方法 选取确诊的39例费城染色体阴性(philadelphia chromosome,Ph-)的初诊MPN患者为研究对象,其中真性红细胞增多症(polycythemia vera,PV)11例、原发性血小板增多症(essential thrombocythemia,ET)13例、原发性骨髓纤维化(primary myelofibrosis,PMF)15例,收集临床特征,如性别、年龄、白细胞计数(WBC)、血红蛋白(HGB)、血小板计数(PLT)、脾大、栓塞情况;通过荧光定量PCR检测JAK2、MPL和CALR 3种驱动基因的突变情况;采用流式细胞仪检测血清TNF、IFN-γ、IL-10、IL-6、IL-4、IL-2表达水平。结果 ① PV组IFN-γ、IL-10水平显著高于ET组(P < 0.01);PMF组IL-10、IL-6、IL-4水平显著高于ET组(P < 0.01),IL-2水平高于ET组(P < 0.05);3组间TNF水平差异无统计学意义(P>0.05)。不同驱动基因突变组间细胞因子水平差异无统计学意义(P>0.05)。JAK2突变阳性患者中,PV组IFN-γ、IL-10、IL-4、IL-2水平高于ET组(P < 0.05);PMF组IL-10、IL-4、IL-2水平显著高于ET组(P < 0.01);3组间TNF、IL-6水平差异无统计学意义(P>0.05)。②不同亚型对红细胞计数(RBC),血细胞比容(HCT),HGB,PLT,凝血酶原时间(PT)呈现出显著性差异,对WBC,乳酸脱氢酶(LDH),活化部分凝血活酶时间(APTT),纤维蛋白原(FIB)未表现出显著性差异。PV组RBC、HCT、HGB水平显著高于PMF和ET组(P < 0.01),ET组PLT水平高于PV组(P < 0.05),PMF组PT长于PV组(P < 0.05)。在PV中,IFN-γ与HCT(r=-0.620),HGB(r=-0.747)呈负相关,TNF与WBC呈负相关(r=-0.679),IL-6与HGB呈负相关(r=-0.678),IL-4与FIB呈负相关(r=-0.743);PMF中,IFN-γ与WBC呈正相关(r=0.575),IL-6与PT呈正相关(r=0.744)。③PV患者更易出现血栓(P < 0.01),PMF患者更易出现肝大(P < 0.05)、脾大(P < 0.01)。④PMF患者国际预后积分系统评分高危组IL-6水平高于低危组(P < 0.05)。结论 MPN不同亚型具有不同的细胞因子表达谱,在疾病发生、发展中起着至关重要的作用,可作为与疾病危险性分层及预后评估相关的新指标以及成为潜在的治疗靶点。Abstract: Objective To explore the expression profile of serum cytokines(tumor necrosis factors[TNF], interferon-γ[IFN-γ], interleukin[IL] -10, IL-6, IL-4 and IL-2)and the role of cytokines in disease evolutionary progression in real-world patients with classical myeloproliferative neoplasms(MPN) of different subtypes and driver mutation states.Methods A total of 39 patients with Philadelphia chromosome-negative(Ph-) newly diagnosed MPN(11 cases of polycythemia vera[PV], 13 cases of essential thrombocythemia[ET], 15 cases of primary myelofibrosis[PMF]) were collected clinical characteristics, such as gender, age, white blood cell count, hemoglobin, platelet count, spleen, and embolism, by quantitative PCR to detect JAK2, MPL and CALR, flow cytometry for serum TNF, IFN-γ, IL-10, IL-6, IL-4, and IL-2 expression levels.Results ① IFN-γ and IL-10 were significantly higher in PV than those in ET(P < 0.01), in PMF, IL-10, IL-6, and IL-4 levels were significantly higher than those in ET(P < 0.01), IL-2 levels were higher than that in ET(P < 0.05); There was no significant difference in TNF level between the three groups(P>0.05). The cytokine levels differed between driver mutation groups were not statistically significant(P>0.05). Among the JAK2 mutation-positive patients, the levels of IFN-γ, IL-10, IL-4, and IL-2 were higher in the PV group than those in the ET group(P < 0.05), The levels of IL-10, IL-4, and IL-2 were significantly higher in the PMF group than those in the ET group(P < 0.01), and there was no significant difference in TNF and IL-6 levels between the three groups(P>0.05). ②The different subtypes showed significant differences for RBC, HCT, HGB, PLT, and PT, but not for WBC, LDH, APTT, or FIB. RBC, HCT, and HGB levels were significantly higher in the PV group than those in the PMF and ET groups(P < 0.01), PLT levels were higher in the ET group than that in the PV group(P < 0.05), and PT time was longer in the PMF group than that in the PV group(P < 0.05). In PV, IFN-γ was negatively correlated with HCT(r=-0.620), HGB(r=-0.747), TNF with WBC(r=-0.679), IL-6 with HGB(r=-0.678), IL-4 with FIB(r=-0.743); in PMF, IFN-γ was positively correlated with WBC(r=0.575), and IL-6 was positively correlated with PT(r=0.744). ③Patients with PV were more likely to have thrombosis(P < 0.01), and patients with PMF were more likely to have hepatomegaly(P < 0.05) and splenomegaly(P < 0.01). ④PMF patients with International Prognostic Scoring System scores had higher IL-6 levels in the high-risk group than that in the low-risk group(P < 0.05).Conclusion Different subtypes of MPN had different cytokine expression profiles, which may play a crucial role in disease development, and may be used as a new indicator related to disease risk stratification and prognosis assessment, and as a potential therapeutic target.
-
Key words:
- myeloproliferative neoplasms /
- cytokines /
- driver gene mutations
-

-
表 1 不同类型MPN患者细胞因子水平比较
pg/mL,M(P25,P75) MPNs 例数 IFN-γ TNF IL-10 IL-6 IL-4 IL-2 PV 11 10.70
(4.80,30.20)2)5.60
(4.30,7.40)2.90
(2.50,5.80)2)6.30
(3.90,10.10)3.20
(1.90,5.60)2.90
(2.70,3.90)PMF 15 6.50
(3.50,14.20)5.60
(3.10,9.60)3.30
(2.90,12.90)2)20.50
(7.90,24.20)2)3.90
(2.90,10.80)2)3.60
(2.90,4.90)1)ET 13 3.10
(0.20,4.60)2.70
(0.20,7.50)1.10
(0.90,1.60)3.85
(1.80,8.20)1.00
(0.50,2.60)1.20
(0.20,2.90)与ET比较,1) P < 0.05,2) P < 0.01。 表 2 JAK2突变阳性的不同亚型MPN患者细胞因子水平比较
pg/mL,M(P25,P75) MPNs 例数 IFN-γ TNF IL-10 IL-6 IL-4 IL-2 PV 10 11.75
(6.30,32.20)1)5.35
(4.20,9.70)2.90
(2.40,5.10)1)6.95
(3.60,10.80)3.40
(1.80,5.70)1)2.90
(2.70,4.80)1)PMF 14 8.45
(3.50,14.40)5.25
(3.00,9.60)4.75
(2.90,13.10)2)15.20(7.10,26.00) 4.35
(3.00,10.80)2)3.75
(3.00,5.20)2)ET 7 2.20
(0.20,3.50)2.40
(0.20,6.90)1.10
(0.90,1.30)4.10
(1.80,8.50)1.00
(0.40,1.30)1.10
(0.20,1.70)与ET比较,1) P < 0.05,2) P < 0.01。 表 3 不同类型MPN患者实验室指标比较
M(P25,P75) 实验室指标 PV(n =11) PMF(n =15) ET(n =13) H P WBC/(×109/L) 8.4(5.6,15.8) 13.4(5.5,20.3) 10.6(8.8,15.1) 1.453 0.484 RBC/(×1012/L) 6.70(6.3,7.8) 3.18(2.4,4.7) 4.63(4.0,5.2) 21.636 < 0.001 HCT/(L/L) 0.615(0.5,0.7) 0.252(0.2,0.4) 0.424(0.4,0.4) 24.404 < 0.001 HGB/(g/L) 197(177.0,204.0) 75(58.0,125.0) 143(118.5,150.5) 24.244 < 0.001 PLT(/×109/L) 409(155.0,527.0) 507(248.0,983.0) 849(665.5,1004.5) 7.686 0.021 LDH(IU/L) 304.8(270.3,351.9) 372.7(270.8,524.4) 287.8(229.1,389.2) 2.523 0.283 PT/s 12.1(11.7,13.1) 13.5(12.5,13.9) 12.9(12.2,13.3) 8.939 0.011 APTT/s 40.3(38.1,47.0) 36.5(35.3,42.5) 39.9(37.5,44.2) 5.500 0.064 FIB/(g/L) 2.38(2.2,2.6) 2.67(2.3,3.0) 2.38(2.0,3.8) 1.127 0.569 表 4 不同亚型MPN患者临床特征比较
例 临床特征 PV(n =11) PMF(n =15) ET(n =13) 合计 χ2 P 血栓 有 7 3 1 11 10.019 0.007 无 4 12 12 28 肝大 有 0 4 0 4 7.131 0.028 无 11 11 13 35 脾大 有 7 12 1 20 15.510 < 0.001 无 4 3 12 19 -
[1] Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia[J]. Blood, 2016, 127(20): 2391-405.
[2] Masselli E, Pozzi G, Gobbi G, et al. Cytokine Profiling in Myeloproliferative Neoplasms: Overview on Phenotype Correlation, Outcome Prediction, and Role of Genetic Variants[J]. Cells, 2020, 9(9)2136. doi: 10.3390/cells9092136
[3] Chatain N, Koschmieder S, Jost E. Role of inflammatory factors during disease pathogenesis and stem cell transplantation in myeloproliferative neoplasms[J]. Cancers, 2020, 12(8): 2250. doi: 10.3390/cancers12082250
[4] Jutzi JS, Mullally A. Remodeling the Bone Marrow Microenvironment-A Proposal for Targeting Pro-inflammatory Contributors in MPN[J]. Front Immunol, 2020, 11: 2093. doi: 10.3389/fimmu.2020.02093
[5] Fisher DAC, Fowles JS, Zhou A, et al. Inflammatory Pathophysiology as a Contributor to Myeloproliferative Neoplasms[J]. Front Immunol, 2021, 12: 683401. doi: 10.3389/fimmu.2021.683401
[6] Ramanathan G, Fleischman AG. The Microenvironment in Myeloproliferative Neoplasms[J]. Hematol Oncol Clin North Am, 2021, 35(2): 205-216. doi: 10.1016/j.hoc.2020.11.003
[7] 黄继贤. MPNs分子遗传学与免疫特征及芦可替尼改善骨纤机制的探讨[D]. 南方医科大学, 2019.
[8] Mendez Luque LF, Blackmon AL, Ramanathan G, et al. Key role of inflammation in myeloproliferative neoplasms: instigator of disease initiation, progression. And symptoms[J]. Curr Hematol Malig Rep, 2019, 14(3): 145-153. doi: 10.1007/s11899-019-00508-w
[9] Zeeh FC, Meyer SC. Current Concepts of Pathogenesis and Treatment of Philadelphia Chromosome-Negative Myeloproliferative Neoplasms[J]. Hamostaseologie, 2021, 41(3): 197-205. doi: 10.1055/a-1447-6667
[10] 刘苗苗, 郭涛. 骨髓增殖性肿瘤血栓事件的治疗现状[J]. 临床血液学杂志, 2021, 34(1): 9-12. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXZ202101003.htm
[11] Merchant S. The JAK2 mutation[J]. Int Rev Cell Mol Biol, 2021, 365: 117-162.
[12] 马骏, 瞿文, 陶景莲, 等. IL-9和IL-6在BCR-ABL-MPN患者中的表达及其意义[J]. 中国实验血液学杂志, 2020, 28(5): 1661-1667. https://www.cnki.com.cn/Article/CJFDTOTAL-XYSY202005043.htm
[13] 包维莺, 施晴, 霍雨佳, 等. IL-2R、IL-6、IL-8、TNF-α在弥漫性大B细胞淋巴瘤中的变化及其意义[J]. 临床血液学杂志, 2023, 36(1): 33-38. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXZ202301007.htm
[14] Baldauf CK, Müller P, Haage TR, et al. Anti-IL-6 cytokine treatment has no impact on elevated hematocrit or splenomegaly in a polycythemia vera mouse model[J]. Blood Adv, 2022, 6(2): 399-404. http://www.sciencedirect.com/science/article/pii/S2473952921006248
[15] 陈朴, 马艳婷, 陈楠, 等. 炎症相关细胞因子与Ph阴性骨髓增殖性肿瘤的相关性[J]. 检验医学, 2021, 36(2): 167-172. https://www.cnki.com.cn/Article/CJFDTOTAL-SHYY202102011.htm
[16] Grabek J, Straube J, Bywater M, et al. MPN: The Molecular Drivers of Disease Initiation, Progression and Transformation and their Effect on Treatment[J]. Cells, 2020, 9(8)1901. http://www.socolar.com/Article/Index?aid=100083633976&jid=100000036184
-




 下载:
下载: