Selinexor combined with PD-1 monoclonal antibody in the treatment of relapsed/refractory primary central nervous system lymphoma: a case report
-
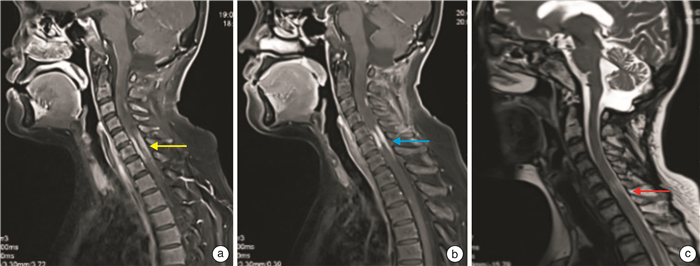
摘要: 为探索复发难治性原发中枢神经系统淋巴瘤(R/R PCNSL)的挽救治疗方案,并为改善该类患者的生存预后提供参考,现回顾1例R/R PCNSL患者的临床病理资料及诊治经过。患者,女,44岁,于外院明确诊断为原发中枢神经系统弥漫大B细胞淋巴瘤非生发中心细胞来源。经多线治疗,疾病未能有效缓解,呈现多药耐药,给予“塞利尼索联合PD-1单抗”挽救治疗,3个周期后达未确定的完全缓解,共接受该方案6个周期,获得无进展生存6个月余。
-
关键词:
- 原发中枢神经系统淋巴瘤 /
- 弥漫大B细胞淋巴瘤 /
- 塞利尼索 /
- XPO1
Abstract: In order to explore the salvage treatment regimen of relapsed/refractory primary central nervous system lymphoma(R/R PCNSL), and provide a reference for improving the survival prognosis of such patients, the clinical pathological data and diagnosis and treatment of a patient with R/R PCNSL were reviewed. A 44-year-old female patient was definitely diagnosed with primary diffuse large B-cell lymphoma of the central nervous system of non-germinal center B cell like in another hospital. After multiple lines of treatment, the disease failed to alleviate effectively and presented with multidrug resistance. The salvage therapy was administrated with selinexor combined with PD-1 monoclonal antibody, and undetermined complete remission was achieved after 3 cycles therapy. Finally, the patient received a total of 6 cycles of this regimen and achieved progression-free survival for more than 6 months. -

-
表 1 患者淋巴瘤相关93基因测序结果
基因名称 碱基改变 突变频率/% CDKN2A c.221dupA 20.3 CARD11 c.1876G>A 19.4 B2M c.20T>Gp.L7 32.9 MLL c.3629C>T 52.4 CARD11 c.2081C>T 49.2 PCLO c.1531C>T 47.9 MEF2B c.247G>A 17.2 CARD11 c.1081T>A 17.1 CCND2 c.841C>T 16.0 CARD11 c.704G>C 11.5 IGH-BCL6 基因重排 15.7 -
[1] 周沙, 曾韫璟, 刘红云, 等. 新药在原发中枢神经系统淋巴瘤诱导和维持治疗的诊疗体会[J]. 临床血液学杂志, 2022, 35(9): 680-684. https://lcxy.whuhzzs.com/article/doi/10.13201/j.issn.1004-2806.2022.09.015
[2] Langner-Lemercier S, Houillier C, Soussain C, et al. Primary CNS lymphoma at first relapse/progression: characteristics, management, and outcome of 256 patients from the French LOC network[J]. Neuro Oncol, 2016, 18(9): 1297-1303. doi: 10.1093/neuonc/now033
[3] Korfel A, Schlegel U. Diagnosis and treatment of primary CNS lymphoma[J]. Nat Rev Neurol, 2013, 9(6): 317-327. doi: 10.1038/nrneurol.2013.83
[4] Mendez JS, Ostrom QT, Gittleman H, et al. The elderly left behind-changes in survival trends of primary central nervous system lymphoma over the past 4 decades[J]. Neuro Oncol, 2018, 20(5): 687-694. doi: 10.1093/neuonc/nox187
[5] Villano JL, Koshy M, Shaikh H, et al. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma[J]. Br J Cancer, 2011, 105(9): 1414-1418. doi: 10.1038/bjc.2011.357
[6] Yin W, Xia X, Wu M, et al. The impact of BCL-2/MYC protein expression and gene abnormality on primary central nervous system diffuse large B-cell lymphoma[J]. Int J Clin Exp Pathol, 2019, 12(6): 2215-2223.
[7] Soussain C, Choquet S, Blonski M, et al. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: Final analysis of the phase Ⅱ 'proof-of-concept' iLOC study by the Lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network[J]. Eur J Cancer, 2019, 117: 121-130. doi: 10.1016/j.ejca.2019.05.024
[8] Lenz G, Davis RE, Ngo VN, et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma[J]. Science, 2008, 319(5870): 1676-1679. doi: 10.1126/science.1153629
[9] Grommes C, Pastore A, Palaskas N, et al. Ibrutinib unmasks critical role of bruton tyrosine kinase in primary CNS lymphoma[J]. Cancer Discov, 2017, 7(9): 1018-1029. doi: 10.1158/2159-8290.CD-17-0613
[10] Montesinos-Rongen M, Schmitz R, Brunn A, et al. Mutations of CARD11 but not TNFAIP3 may activate the NF-κB pathway in primary CNS lymphoma[J]. Acta Neuropathol, 2010, 120(4): 529-535. doi: 10.1007/s00401-010-0709-7
[11] Azmi AS, Uddin MH, Mohammad RM. The nuclear export protein XPO1-from biology to targeted therapy[J]. Nat Rev Clin Oncol, 2021, 18(3): 152-169. doi: 10.1038/s41571-020-00442-4
[12] Wang AY, Liu HT. The past, present, and future of CRM1/XPO1 inhibitors[J]. Stem Cell Investig, 2019, 6: 6. doi: 10.21037/sci.2019.02.03
[13] Kasamon YL, Price LSL, Okusanya OO, et al. FDA approval summary: selinexor for relapsed or refractory diffuse large B-cell lymphoma[J]. Oncologist, 2021, 26(10): 879-886. doi: 10.1002/onco.13859
[14] Kuruvilla J, Savona M, Baz R, et al. Selective inhibition of nuclear export with selinexor in patients with non-Hodgkin lymphoma[J]. Blood, 2020, 136(2): 259. doi: 10.1182/blood.2020007232
[15] Lassman AB, Wen PY, van den Bent MJ, et al. A phase Ⅱ study of the efficacy and safety of oral selinexor in recurrent glioblastoma[J]. Clin Cancer Res, 2022, 28(3): 452-460. doi: 10.1158/1078-0432.CCR-21-2225
[16] Bobillo S, P Abrisqueta, C Carpio, et al. Promising activity of selinexor in the treatment of a patient with refractory diffuse large B-cell lymphoma and central nervous system involvement[J]. Haematologica, 2018, 103(2): e92-e93. doi: 10.3324/haematol.2017.181636
[17] Crespo M, Carabia J, Jiménez I, et al. XPO1 inhibition by selinexor synergizes with BCR inhibition, blocks tumor growth and prolongs survival in a bioluminescent animal model of primary central nervous system lymphoma[J]. Blood, 2016, 128(22): 463. doi: 10.1182/blood.V128.22.463.463
[18] Farren MR, Hennessey RC, Shakya R, et al. The exportin-1 inhibitor selinexor exerts superior antitumor activity when combined with T-cell checkpoint inhibitors[J]. Mol Cancer Ther, 2017, 16(3): 417-427. doi: 10.1158/1535-7163.MCT-16-0498
[19] Kapoor I, Li Y, Sharma A, et al. Resistance to BTK inhibition by ibrutinib can be overcome by preventing FOXO3a nuclear export and PI3K/AKT activation in B-cell lymphoid malignancies[J]. Cell Death Dis, 2019, 10(12): 924. doi: 10.1038/s41419-019-2158-0
[20] Fischer MA, Friedlander SY, Arrate MP, et al. Venetoclax response is enhanced by selective inhibitor of nuclear export compounds in hematologic malignancies[J]. Blood Adv, 2020, 4(3): 586-598. doi: 10.1182/bloodadvances.2019000359
[21] Li L, Zhang J, Chen J, et al. B-cell receptor-mediated NFATc1activation induces IL-10/STAT3/PD-L1 signaling in diffuse large B-cell lymphoma[J]. Blood, 2018, 132(17): 1805-1817. doi: 10.1182/blood-2018-03-841015
[22] Oestreich KJ, Yoon H, Ahmed R, et al. NFATc1regulates PD-1 expression upon T cell activation[J]. J Immunol, 2008, 181(7): 4832-4839. doi: 10.4049/jimmunol.181.7.4832
[23] Tai YT, Landesman Y, Acharya C, et al. CRM1 inhibition induces tumor cell cytotoxicity and impairs osteoclastogenesis in multiple myeloma: molecular mechanisms and therapeutic implications[J]. Leukemia, 2014, 28(1): 155-165. doi: 10.1038/leu.2013.115
-




 下载:
下载: