Self-reported health-related quality of life and associated variables in adult survivors of childhood chronic myeloid leukemia
-
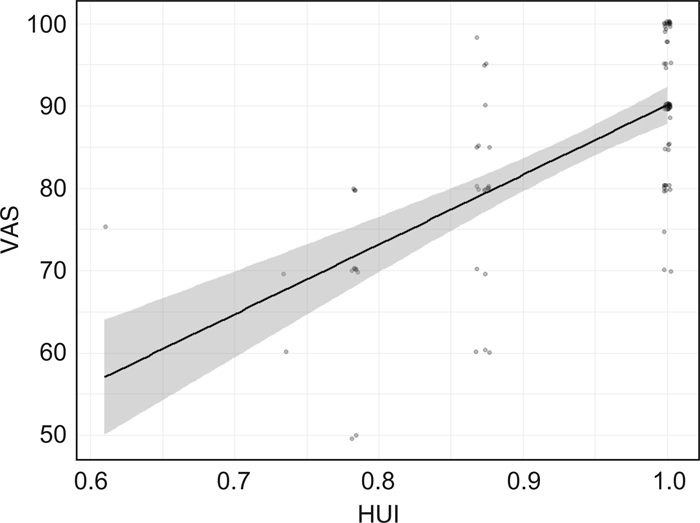
摘要: 目的 评估酪氨酸激酶抑制剂(tyrosine kinase inhibitor,TKI)治疗的慢性粒细胞白血病(chronic myeloid leukemia,CML)儿童成年后幸存者自我报告的生活质量及其影响因素。方法 2020年9月—2021年12月在全国范围内向发病年龄 < 18岁、目前年龄≥18岁的CML患者发放调查问卷,方式包括电子问卷及纸质版问卷。该问卷包括3个部分:①受访者人口学、疾病及诊疗特征;②参考中国居民膳食指南2016版的生活方式调查;③采用EQ-5D-3L评估生活质量量表[包括健康效用值(health utility index,HUI)、视觉模拟尺度评分(visual analogue scale,VAS)]。结果 共有93例CML儿童成年后受访者符合标准并被纳入本研究。CML儿童成年后受访者生活质量下降的主要表现为疼痛/不舒服(躯体功能)和焦虑/抑郁(心理功能)。对HUI进行多因素分析显示,女性(AOR=0.130,P=0.018)、药物相关不良反应越多(AOR=0.493,P=0.008)、睡眠时间不达标(AOR=0.176,P=0.033)者,HUI得分越低。对VAS进行多因素分析显示,诊断年龄越小(B=1.636,P=0.040)、目前年龄越大(B=-2.071,P=0.007)、TKI服用时间短(B=0.167,P=0.007)、不良反应个数越多(B=-1.908,P=0.012)、运动量不达标(B=-5.895,P=0.025)、吸烟者(B=-8.624,P=0.023),VAS得分越低。健康生活方式个数与HUI(r=0.536,P=3.14×10-8)、VAS(r=0.391,P=1.06×10-4)呈中度相关。结论 CML儿童成年后受访者生活质量下降的主要表现为躯体功能和心理功能。女性、TKI服用时间短、药物不良反应越多、运动量不达标、吸烟者,生活质量越差。此外,健康生活方式越多,生活质量越好。Abstract: Objective To explore the health-related quality of life(HRQoL) and associated variables in adult survivors of childhood chronic myeloid leukemia(CML) receiving tyrosine kinase inhibitors(TKI).Methods A cross-sectional questionnaire was given to adult survivors of childhood CML, who were < 18 years at the diagnosis of CML and ≥18 years at the study. The questionnaire consisted of 3 parts: demographic information and clinical information, the lifestyle behaviors survey of the Dietary Guidelines for Chinese(2016) and the Chinese version of EQ-5D-3L as HRQoL questionnaire, which includes health utility index(HUI) and visual analogue scale(VAS).Results A total of 93 adults with childhood CML were included in the study. The repaired HRQoL was mainly manifested in pain/discomfort(physical functioning) and anxiety/depression(mental functioning). Multivariate analysis of HUI showed that females(AOR=0.130, P=0.018), with more drug-related adverse events(AOR=0.493, P=0.008), with less sleep time(AOR=0.176, P=0.033) were associated with lower HUI score. Multivariate analysis of VAS showed that the younger children at diagnosis(B=1.636, P=0.040), the older adults at study(B=-2.071, P=0.007), with shorter TKI-therapy duration(B=0.167, P=0.007), with more drug-related adverse events(B=-1.908, P=0.012), substandard physical activity(B=-5.895, P=0.025), smoking(B=-8.624, P=0.023) were associated with lower VAS score. The number of healthy lifestyles was moderately correlated with HUI(r=0.536, P=3.14×10-8) and VAS(r=0.391, P=1.06×10-4), respectively.Conclusion Females, with younger age at diagnosis, and older age at study, the more drug-related adverse events, shorter TKI-therapy duration, less sleep time, less physical activity, and smoking, the worse HRQoL they had. In addition, the more healthy lifestyle, the higher HRQoL.
-
Key words:
- chronic myeloid leukemia /
- childhood /
- adult /
- health-related quality of life
-

-
表 1 93例CML儿童成年后受访者的基本特征
特征 受访者(n=93) 男性/例(%) 55(59.1) 中位诊断年龄(范围)/岁 16(5~17) 中位目前年龄(范围)/岁 22(18~37) 目前状态/例(%) 上学 50(53.8) 工作 43(46.2) 学历/例(%) < 大学 31(33.3) ≥大学 62(66.7) 存在不良反应/例(%) 40(43.0) 中位TKI总服用时间(范围)/月 84(7~240) 分子学反应MR4.0/例(%) ≥MR4.0 46(49.5) < MR4.0 47(50.5) 目前口服TKI种类/例(%) 原研伊马替尼 35(37.6) 仿制伊马替尼 26(28.0) 达沙替尼 10(10.8) 尼洛替尼 16(17.2) 其他 6(6.5) 中位饮食合格个数(范围) 6(0~11) 运动合格情况/例(%) 42(45.2) 睡眠合格情况/例(%) 68(73.1) 吸烟情况/例(%) 自己和周围都不吸烟 45(48.4) 自己不吸但周围吸烟 39(41.9) 自己吸烟 9(9.7) 饮酒情况/例(%) 不饮酒 70(75.3) 适量饮酒 22(23.7) 有害饮酒 1(1.1) 中位BMI值(范围)/(kg/m2) 21.31(15.43~36.71) BMI分组/例(%) 正常 65(69.9) 消瘦 8(8.6) 超重 14(15.1) 肥胖 6(6.5) 表 2 93例CML儿童成年后受访者基于EQ-5D-3L量表的生活质量的单因素分析
组别 例数 HUI VAS X±S 检验值 P X±S 检验值 P 性别 3.486 0.062 1.217 0.270 男 55 0.953±0.082 85.873±11.788 女 38 0.914±0.100 83.237±11.913 诊断年龄 0.234 0.629 0.199 0.656 < 16岁 43 0.935±0.086 84.395±11.937 ≥16岁 50 0.939±0.096 85.140±11.879 目前年龄 0.041 0.840 0.001 0.978 < 22岁 52 0.937±0.089 85.595±9.771 ≥22岁 41 0.937±0.095 84.137±13.378 目前状态 0.125 0.723 1.350 0.245 上学 50 0.941±0.087 85.980±11.804 工作 43 0.932±0.096 83.419±11.885 学历 5.929 0.015 0.285 0.593 < 大学 31 0.907±0.100 83.710±12.757 ≥大学 62 0.952±0.084 85.339±11.433 不良反应 11.716 0.001 10.178 0.001 有 40 0.901±0.108 80.756±11.745 无 53 0.966±0.063 87.981±11.023 TKI总服用时间 1.591 0.207 1.180 0.277 < 84个月 52 0.927±0.092 83.545±11.874 ≥84个月 41 0.947±0.090 85.918±11.830 分子学反应MR4.0 1.297 0.255 3.249 0.071 ≥MR4.0 46 0.925±0.080 86.723±11.536 < MR4.0 47 0.947±0.101 82.826±11.960 目前口服TKI 8.201 0.084 12.370 0.015 原研伊马替尼 35 0.967±0.070 90.114±8.235 仿制伊马替尼 26 0.902±0.108 81.115±10.823 达沙替尼 10 0.925±0.091 82.455±14.794 尼洛替尼 16 0.930±0.090 82.375±12.868 其他 6 0.957±0.097 79.600±18.528 饮食合格个数 8.217 0.004 8.507 0.004 < 6个 55 0.903±0.102 79.947±13.531 ≥6个 38 0.961±0.075 88.145±9.256 运动情况 16.166 < 0.001 18.760 < 0.001 合格 42 0.981±0.046 80.157±12.059 不合格 51 0.901±0.103 90.429±8.849 睡眠情况 6.067 0.014 0.714 0.398 合格 68 0.952±0.082 85.662±10.720 不合格 25 0.897±0.104 82.440±14.466 吸烟情况 6.909 0.009 8.124 0.004 不吸烟 45 0.946±0.084 86.024±11.168 吸烟 48 0.852±0.119 73.333±12.500 饮酒情况 0.096 0.756 0.031 0.860 不饮酒 70 0.938±0.083 85.257±10.896 饮酒 23 0.937±0.094 83.391±14.553 BMI分组 2.084 0.353 5.944 0.051 正常 65 0.946±0.088 86.754±10.680 消瘦 8 0.924±0.112 82.500±15.811 超重或肥胖 20 0.914±0.094 79.350±12.516 表 3 93例CML儿童成年后受访者基于EQ-5D-3L量表的生活质量的多因素分析
因素 HUI VAS AOR 95%CI P B 标准误 t P 女(ref.男) 0.130 0.020~0.631 0.018 -1.065 2.339 -0.455 0.650 诊断年龄≥16岁(ref. < 16岁) 1.458 0.854~2.975 0.200 1.636 0.783 2.091 0.040 目前年龄≥22岁(ref. < 22岁) 0.765 0.373~1.264 0.344 -2.071 0.747 -2.772 0.007 工作(ref.上学) 1.234 0.162~10.672 0.841 1.491 2.951 0.505 0.615 教育程度 < 大学(ref.≥大学) 0.185 0.026~0.961 0.061 3.030 2.450 1.237 0.220 不良反应个数 0.493 0.272~0.798 0.008 -1.908 0.741 -2.575 0.012 TKI总服用时间 1.033 0.993~1.097 0.158 0.167 0.061 2.760 0.007 已达MR4.0(ref.未达MR4.0) 0.867 0.194~3.811 0.848 1.728 2.113 0.818 0.416 原研伊马替尼(ref.仿制伊马替尼) 3.350 0.613~21.669 0.175 4.080 2.846 1.433 0.156 达沙替尼(ref.仿制伊马替尼) 1.146 0.083~15.414 0.916 -0.929 3.948 -0.235 0.815 尼洛替尼(ref.仿制伊马替尼) 0.404 0.046~3.110 0.390 -3.312 3.389 -0.977 0.332 其他TKI(ref.仿制伊马替尼) 48.001 0.288~59.129 0.280 -2.724 5.643 -0.483 0.631 饮食合格数 < 6(ref.合格数≥6) 0.430 0.104~1.677 0.227 -4.582 2.363 1.939 0.056 运动情况不合格(ref.合格) 0.323 0.065~1.427 0.143 -5.859 2.568 2.281 0.025 睡眠时间不合格(ref.合格) 0.176 0.033~0.838 0.033 0.228 2.610 -0.087 0.931 吸烟(ref.不吸烟) 0.443 0.035~3.965 0.482 -8.624 3.712 -2.323 0.023 饮酒(ref.不饮酒) 0.203 0.030~1.139 0.078 -0.261 2.551 0.102 0.919 BMI消瘦(ref.正常) 0.291 0.027~3.361 0.300 -4.143 4.005 -1.035 0.304 BMI超重或肥胖(ref.正常) 0.212 0.023~1.441 0.133 -4.272 2.661 -1.606 0.113 -
[1] 黎纬明, 余甜. 慢性髓系白血病合并传染病的规范化管理进展[J]. 临床血液学杂志, 2023, 36(5): 316-320. doi: 10.13201/j.issn.1004-2806.2023.05.004 https://lcxy.whuhzzs.com/article/doi/10.13201/j.issn.1004-2806.2023.05.004
[2] Millot F, Guilhot J, Suttorp M, et al. Prognostic discrimination based on the EUTOS long-term survival score within the International Registry for Chronic Myeloid Leukemia in children and adolescents[J]. Haematologica, 2017, 102(10): 1704-1708. doi: 10.3324/haematol.2017.170035
[3] Pulte D, Gondos A, Brenner H. Trends in survival after diagnosis with hematologic malignancy in adolescence or young adulthood in the United States, 1981-2005[J]. Cancer, 2009, 115(21): 4973-4979. doi: 10.1002/cncr.24548
[4] 杨月欣, 张环美. 《中国居民膳食指南(2016)》简介[J]. 营养学报, 2016, 38(3): 209-217. doi: 10.13325/j.cnki.acta.nutr.sin.2016.03.002
[5] Wu C, Gong Y, Wu J, et al. Chinese Version of the EQ-5D Preference Weights: Applicability in a Chinese General Population[J]. PLoS One, 2016, 11(10): e164334.
[6] Golicki D, Niewada M. General population reference values for 3-level EQ-5D(EQ-5D-3L)questionnaire in Poland[J]. Pol Arch Med Wewn, 2015, 125(1-2): 18-26.
[7] Shiroiwa T, Fukuda T, Ikeda S, et al. Japanese population norms for preference-based measures: EQ-5D-3L, EQ-5D-5L, and SF-6D[J]. Qual Life Res, 2016, 25(3): 707-719. doi: 10.1007/s11136-015-1108-2
[8] Yao Q, Liu C, Zhang Y, et al. Population norms for the EQ-5D-3L in China derived from the 2013 National Health Services Survey[J]. J Glob Health, 2021, 11: 8001. doi: 10.7189/jogh.11.08001
[9] Pogany L, Barr RD, Shaw A, et al. Health status in survivors of cancer in childhood and adolescence[J]. Qual Life Res, 2006, 15(1): 143-157. doi: 10.1007/s11136-005-0198-7
[10] Badr H, Chandra J, Paxton RJ, et al. Health-related quality of life, lifestyle behaviors, and intervention preferences of survivors of childhood cancer[J]. J Cancer Surviv, 2013, 7(4): 523-534. doi: 10.1007/s11764-013-0289-3
[11] Halvorsen JF, Sund AM, Zeltzer L, et al. Health-related quality of life and psychological distress in young adult survivors of childhood cancer and their association with treatment, education, and demographic factors[J]. Qual Life Res, 2018, 27(2): 529-537. doi: 10.1007/s11136-017-1716-0
[12] Huang IC, Brinkman TM, Kenzik K, et al. Association between the prevalence of symptoms and health-related quality of life in adult survivors of childhood cancer: a report from the St Jude Lifetime Cohort study[J]. J Clin Oncol, 2013, 31(33): 4242-4251. doi: 10.1200/JCO.2012.47.8867
[13] van Gorp M, van Erp L, Maas A, et al. Increased health-related quality of life impairments of male and female survivors of childhood cancer: DCCSS LATER 2 psycho-oncology study[J]. Cancer, 2022, 128(5): 1074-1084. doi: 10.1002/cncr.34003
[14] Wan PS, Aizuddin AN, Tumian NR, et al. Health-related quality of life using EQ-5D among chronic myeloid leukaemia patients in health centres in Klang Valley, Malaysia[J]. PLoS One, 2021, 16(8): e256804.
[15] Jiang Q, Wang HB, Yu L, et al. Variables associated with patient-reported outcomes in persons with chronic myeloid leukemia receiving tyrosine kinase-inhibitor therapy[J]. J Cancer Res Clin Oncol, 2017, 143(6): 1013-1022. doi: 10.1007/s00432-017-2353-2
[16] Pogany L, Barr RD, Shaw A, et al. Health status in survivors of cancer in childhood and adolescence[J]. Qual Life Res, 2006, 15(1): 143-157. doi: 10.1007/s11136-005-0198-7
[17] Bize R, Johnson JA, Plotnikoff RC. Physical activity level and health-related quality of life in the general adult population: a systematic review[J]. Prev Med, 2007, 45(6): 401-415. doi: 10.1016/j.ypmed.2007.07.017
[18] Gerhardt CA, Vannatta K, Valerius KS, et al. Social and romantic outcomes in emerging adulthood among survivors of childhood cancer[J]. J Adolesc Health, 2007, 40(5): 462-469.
[19] Murnane A, Kiss N, Fraser SF, et al. Health-related quality of life, fatigue and health behaviours in Australian adolescent and young adult cancer survivors[J]. Pediatr Blood Cancer, 2021, 68(10): e29243.
[20] Lemay V, Caru M, Samoilenko M, et al. Physical Activity and Sedentary Behaviors in Childhood Acute Lymphoblastic Leukemia Survivors[J]. J Pediatr Hematol Oncol, 2020, 42(1): 53-60. doi: 10.1097/MPH.0000000000001594
[21] Zhang FF, Ojha RP, Krull KR, et al. Adult Survivors of Childhood Cancer Have Poor Adherence to Dietary Guidelines[J]. J Nutr, 2016, 146(12): 2497-2505. doi: 10.3945/jn.116.238261
[22] Zhang FF, Hudson MM, Huang IC, et al. Lifestyle factors and health-related quality of life in adult survivors of childhood cancer: A report from the St. Jude Lifetime Cohort Study[J]. Cancer, 2018, 124(19): 3918-3923. doi: 10.1002/cncr.31647
[23] Brinksma A, Sanderman R, Roodbol PF, et al. Malnutrition is associated with worse health-related quality of life in children with cancer[J]. Support Care Cancer, 2015, 23(10): 3043-3052. doi: 10.1007/s00520-015-2674-0
[24] Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires[J]. J Clin Epidemiol, 2007, 60(1): 34-42.
-




 下载:
下载: