-
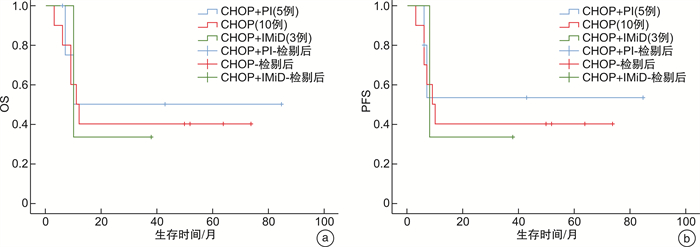
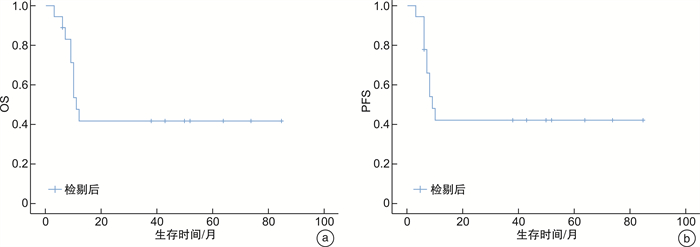
摘要: 目的 总结浆母细胞淋巴瘤(plasmablastic lymphoma,PBL)患者的临床特征和治疗,探讨诊断和预后因素。方法 回顾性分析2015年3月—2021年11月在南京鼓楼医院诊治的18例PBL患者的临床及病理特点、治疗及预后因素。结果 18例患者中位发病年龄为64(44~79)岁,男12例,女6例,HIV感染占5.6%,Ann Arbor分期Ⅲ~Ⅳ期占50.0%,IPI评分中高~高危占38.9%。肿瘤细胞表达CD38、CD138、MUM1,不表达CD20、ALK,2例EBER阳性,Ki67呈高表达。18例患者均接受CHOP样方案一线治疗,5例加用硼替佐米,3例加用来那度胺,2例患者接受局部放疗,3例接受局部手术,1例接受自体造血干细胞移植。治疗后达完全缓解(complete remission,CR)8例(44.4%),总有效率(overall response rate,ORR)为77.5%。IPI低~中低危组7例(63.6%)达CR,优于中高~高危组的1例(14.3%)(P=0.04)。13例CD30阴性患者的ORR (92.3%)优于5例CD30阳性患者的ORR (40.0%)(P=0.017)。18例患者中位随访时间10.5个月,中位无进展生存期(progressive-free survival,PFS)9个月,总生存期(overall survival,OS)11个月。影响OS的独立因素包括分期Ⅲ~Ⅳ(P=0.012)、IPI评分中高~高危(P=0.023)、初始治疗后≥部分缓解(P=0.031)。影响PFS的独立因素包括分期Ⅲ~Ⅳ(P=0.011)、IPI评分中高~高危(P=0.023)、初始治疗后≥部分缓解(P=0.023)。结论 PBL常见于中老年男性,常累及结外组织,Ki67呈高表达,治疗无统一方案,预后较差,IPI指数、疾病分期和初始治疗的反应与预后相关。Abstract: Objective To analyze the clinical characteristics and outcomes of patients with plasmablastic lymphoma(PBL) and to address the prognostic factors.Methods The clinical and pathological characteristics, treatment and prognostic factors of 18 patients with PBL who were diagnosed and treated in Nanjing Drum Tower Hospital from March 2015 to November 2021 were retrospectively analyzed.Results 18 patients included 12 males and 6 females with a median age of 64 years(range 44-79). HIV positivity was 5.6%. Stage Ⅲ-Ⅳ disease was present in 50.0%. A high-risk IPI score was present in 38.9%. All 18 patients were positive for one or more plasma cell markers CD38, CD138, and/or MUM-1 while expression of CD20 and ALK was universally absent. EBER positive was 11.1%. Ki67 was highly expressed. All patients received a CHOP-like regimen, 5 patients received bortezomib, 3 patients received lenalidomide, 2 patients received local radiotherapy, 3 patients received local surgery, and 1 patient received autologous hematopoietic stem cell transplantation. After treatment, 8 cases(44.4%) were complete remission(CR), and the overall response rate(ORR) was 77.5%. 7 cases(63.6%) with low-risk IPI scores were CR while 1 case(14.3%) with high-risk IPI scores(P=0.04). The ORR of 13 cases with CD30 negative was 92.3%, which was better than 40.0% of the 5 cases with CD30 positive(P=0.017). The median follow-up time was 10.5 months, the median progressive-free survival(PFS) was 9 months, and the median overall survival(OS) was 11 months. Independent factors affecting OS included stage Ⅲ-Ⅳ(P=0.012), high-risk IPI score(P=0.023), ≥partial remission after initial treatment(P=0.031). The independent factors affecting PFS included stage Ⅲ-Ⅳ(P=0.011), high-risk IPI score(P=0.023), ≥partial remission after initial treatment(P=0.023).Conclusion PBL mostly occurs in middle-age and elderly males, with extranodal tissue involved, high expression of Ki67, and poor prognosis. There is no standard treatment regimen for PBL. The IPI score, disease stage, and response to initial treatment are associated with outcomes.
-
Key words:
- plasmablastic lymphoma /
- clinical features /
- treatment /
- prognosis
-

-
表 1 18例患者的临床特征与疗效
临床特征 CR/例 未达CR/例 P ≥PR/例 未达PR/例 P CHOP 4 6 0.698 7 3 0.543 CHOP+PI 3 2 4 1 CHOP+IMiD 1 2 3 0 CHOP 4 6 0.671 7 3 0.375 CHOP+PI或IMiD 4 4 7 1 Ⅰ~Ⅱ期 2 7 0.058 6 3 0.257 Ⅲ~Ⅳ期 6 3 8 1 IPI评分低~中低危组 7 4 0.040 10 1 0.093 IPI评分中高~高危组 1 6 4 3 CD79a阳性 6 7 0.814 11 2 0.261 CD79a阴性 2 3 3 2 CD30阳性 1 4 0.196 2 3 0.017 CD30阴性 7 6 12 1 MYC≥40% 3 4 0.447 4 3 0.185 MYC<40% 5 3 7 1 Ki67≥80% 5 6 0.914 8 3 0.518 Ki67 < 80% 3 4 6 1 IFE单克隆 1 3 0.375 3 1 0.880 IFE多克隆 7 7 11 3 表 2 患者的临床特征与预后分析
临床特征 OS PFS P HR 95%CI P HR 95%CI 性别(男vs女) 0.656 0.734 0.188~2.861 0.588 0.687 0.177~2.674 年龄(≥60岁vs < 60岁) 0.792 0.833 0.215~3.228 0.796 0.836 0.216~3.242 原发部位(结外vs淋巴结) 0.783 0.803 0.169~3.818 0.774 0.795 0.167~3.790 分期(Ⅲ~Ⅳ vsⅠ~Ⅱ) 0.012 6.230 1.507~25.762 0.011 6.426 1.528~27.021 LDH(升高vs正常) 0.192 2.328 0.655~8.273 0.182 2.370 0.667~8.418 IFE(单克隆vs多克隆) 0.507 0.630 0.161~2.469 0.426 0.575 0.147~2.249 IPI评分(中高~高危组vs低~中低危组) 0.023 4.538 1.230~16.739 0.023 4.587 1.229~17.123 CD79a(阳性vs阴性) 0.636 0.720 0.184~2.809 0.616 0.705 0.180~2.766 CD30(阳性vs阴性) 0.124 2.723 0.760~9.751 0.127 2.708 0.754~9.720 Ki67(≥80% vs < 80%) 0.709 1.274 0.357~4.539 0.770 1.209 0.339~4.321 治疗(CHOP样vs CHOP+PI vs CHOP+IMiD) 0.870 0.935 0.417~2.097 0.892 0.946 0.423~2.116 初始疗效(CR vs未达CR) 0.068 0.008 0~1.440 0.068 0.007 0~1.431 初始疗效(≥PR vs未达PR) 0.031 0.233 0.062~0.873 0.023 0.214 0.056~0.811 -
[1] Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms[J]. Blood, 2016, 127(20): 2375-2390. doi: 10.1182/blood-2016-01-643569
[2] Li J, Zhao S, Wang JX, et al. CD20-negative diffuse large B cell lymphoma: a comprehensive analysis of 695 cases[J]. Tumour Biol, 2016, 37(3): 3619-3637. doi: 10.1007/s13277-015-4205-5
[3] 刘果, 刘锋. 头颈部T淋巴母细胞淋巴瘤临床诊治分析[J]. 临床耳鼻咽喉头颈外科杂志, 2021, 35(6): 551-554. doi: 10.13201/j.issn.2096-7993.2021.06.015
[4] Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma[J]. Blood, 2015, 125(15): 2323-2330. doi: 10.1182/blood-2014-10-567479
[5] Castillo JJ, Winer ES, Stachurski D, et al. Clinical and pathological differences between human immunodeficiency virus-positive and human immunodeficiency virus-negative patients with plasmablastic lymphoma[J]. Leuk Lymphoma, 2010, 51(11): 2047-2053. doi: 10.3109/10428194.2010.516040
[6] Yap DRY, Tan GF, Chang EWY, et al. Clinical features of plasmablastic lymphoma: case series from an Asian tertiary cancer center and literature review[J]. J Hematol, 2020, 9(3): 71-78. doi: 10.14740/jh672
[7] 姜骁娜, 史雪, 侯峰, 等. 7例浆母细胞淋巴瘤临床特征分析[J]. 临床血液学杂志, 2022, 35(1): 63-67. https://lcxy.whuhzzs.com/article/doi/10.13201/j.issn.1004-2806.2022.01.012
[8] 杜建伟, 孙星, 李钢苹, 等. 九例人免疫缺陷病毒阴性浆母细胞淋巴瘤患者临床特征分析[J]. 中华血液学杂志, 2016, 37(12): 1077-1080. doi: 10.3760/cma.j.issn.0253-2727.2016.12.013
[9] Tchernonog E, Faurie P, Coppo P, et al. Clinical characteristics and prognostic factors of plasmablastic lymphoma patients: analysis of 135 patients from the LYSA group[J]. Ann Oncol, 2017, 28(4): 843-848. doi: 10.1093/annonc/mdw684
[10] Morscio J, Dierickx D, Nijs J, et al. Clinicopathologic comparison of plasmablastic lymphoma in HIV-positive, immunocompetent, and posttransplant patients: single-center series of 25 cases and meta-analysis of 277 reported cases[J]. Am J Surg Pathol, 2014, 38(7): 875-886. doi: 10.1097/PAS.0000000000000234
[11] Vega F, Chang CC, Medeiros LJ, et al. Plasmablastic lymphomas and plasmablastic plasma cell myelomas have nearly identical immunophenotypic profiles[J]. Mod Pathol, 2005, 18(6): 806-815. doi: 10.1038/modpathol.3800355
[12] Valera A, Balagué O, Colomo L, et al. IG/MYC rearrangements are the main cytogenetic alteration in plasmablastic lymphomas[J]. Am J Surg Pathol, 2010, 34(11): 1686-1694. doi: 10.1097/PAS.0b013e3181f3e29f
[13] Chen BJ, Chuang SS. Lymphoid neoplasms with plasmablastic differentiation: a comprehensive review and diagnostic approaches[J]. Adv Anat Pathol, 2020, 27(2): 61-74. doi: 10.1097/PAP.0000000000000253
[14] Ahn JS, Okal R, Vos JA, et al. Plasmablastic lymphoma versus plasmablastic myeloma: an ongoing diagnostic dilemma[J]. J Clin Pathol, 2017, 70(9): 775-780. doi: 10.1136/jclinpath-2016-204294
[15] Mori H, Fukatsu M, Ohkawara H, et al. Heterogeneity in the diagnosis of plasmablastic lymphoma, plasmablastic myeloma, and plasmablastic neoplasm: a scoping review[J]. Int J Hematol, 2021, 114(6): 639-652. doi: 10.1007/s12185-021-03211-w
[16] Castillo J, Pantanowitz L, Dezube BJ. HIV-associated plasmablastic lymphoma: lessons learned from 112 published cases[J]. Am J Hematol, 2008, 83(10): 804-809. doi: 10.1002/ajh.21250
[17] Lopez A, Abrisqueta P. Plasmablastic lymphoma: current perspectives[J]. Blood Lymphatic Cancer, 2018, 8: 63-70. doi: 10.2147/BLCTT.S142814
[18] Harmon CM, Smith LB. Plasmablastic lymphoma: a review of clinicopathologic features and differential diagnosis[J]. Arch Pathol Lab Med, 2016, 140(10): 1074-1078. doi: 10.5858/arpa.2016-0232-RA
[19] Castillo JJ, Furman M, Beltrán BE, et al. Human immunodeficiency virus-associated plasmablastic lymphoma: poor prognosis in the era of highly active antiretroviral therapy[J]. Cancer, 2012, 118(21): 5270-5277. doi: 10.1002/cncr.27551
[20] Schommers P, Wyen C, Hentrich M, et al. Poor outcome of HIV-infected patients with plasmablastic lymphoma: results from the German AIDS-related lymphoma cohort study[J]. AIDS, 2013, 27(5): 842-845. doi: 10.1097/QAD.0b013e32835e069d
[21] Castillo JJ, Winer ES, Stachurski D, et al. Prognostic factors in chemotherapy-treated patients with HIV-associated Plasmablastic lymphoma[J]. Oncologist, 2010, 15(3): 293-299. doi: 10.1634/theoncologist.2009-0304
[22] Makady NF, Ramzy D, Ghaly R, et al. The emerging treatment options of plasmablastic lymphoma: analysis of 173 individual patient outcomes[J]. Clin Lymphoma Myeloma Leuk, 2021, 21(3): e255-e263. doi: 10.1016/j.clml.2020.11.025
[23] Re A, Michieli M, Casari S, et al. High-dose therapy and autologous peripheral blood stem cell transplantation as salvage treatment for AIDS-related lymphoma: long-term results of the Italian Cooperative Group on AIDS and Tumors(GICAT)study with analysis of prognostic factors[J]. Blood, 2009, 114(7): 1306-1313. doi: 10.1182/blood-2009-02-202762
[24] Cattaneo C, Re A, Ungari M, et al. Plasmablastic lymphoma among human immunodeficiency virus-positive patients: results of a single center's experience[J]. Leuk Lymphoma, 2015, 56(1): 267-269. doi: 10.3109/10428194.2014.911867
[25] Castillo JJ, Guerrero-Garcia T, Baldini F, et al. Bortezomib plus EPOCH is effective as frontline treatment in patients with plasmablastic lymphoma[J]. Br J Haematol, 2019, 184(4): 679-682. doi: 10.1111/bjh.15156
[26] Pretscher D, Kalisch A, Wilhelm M, et al. Refractory plasmablastic lymphoma-a review of treatment options beyond standard therapy[J]. Ann Hematol, 2017, 96(6): 967-970. doi: 10.1007/s00277-016-2904-7
[27] Laurent C, Fabiani B, Do C, et al. Immune-checkpoint expression in Epstein-Barr virus positive and negative plasmablastic lymphoma: a clinical and pathological study in 82 patients[J]. Haematologica, 2016, 101(8): 976-984. doi: 10.3324/haematol.2016.141978
[28] Castillo JJ, Reagan JL, Bishop KD, et al. Viral lymphomagenesis: from pathophysiology to the rationale for novel therapies[J]. Br J Haematol, 2014, 165(3): 300-315. doi: 10.1111/bjh.12788
[29] Delmore JE, Issa GC, Lemieux ME, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc[J]. Cell, 2011, 146(6): 904-917. doi: 10.1016/j.cell.2011.08.017
[30] Mine S, Hishima T, Suganuma A, et al. Interleukin-6-dependent growth in a newly established plasmablastic lymphoma cell line and its therapeutic targets[J]. Sci Rep, 2017, 7(1): 10188. doi: 10.1038/s41598-017-10684-5
-

| 引用本文: | 徐勇, 陈兵, 许景艳, 等. 18例浆母细胞淋巴瘤的临床特征及预后分析[J]. 临床血液学杂志, 2023, 36(9): 656-661. doi: 10.13201/j.issn.1004-2806.2023.09.009 |
| Citation: | XU Yong, CHEN Bing, XU Jingyan, et al. Clinical characteristics and outcomes of 18 cases of plasmablastic lymphoma[J]. J Clin Hematol, 2023, 36(9): 656-661. doi: 10.13201/j.issn.1004-2806.2023.09.009 |
- Figure 1.
- Figure 2.



 下载:
下载: