Melphalan combined with autologous transplantation in the treatment of multiple myeloma: a single center clinical analysis of progression-free survival
-
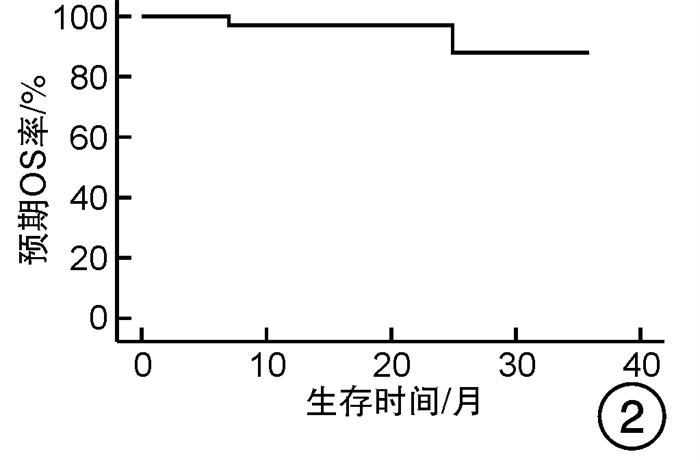
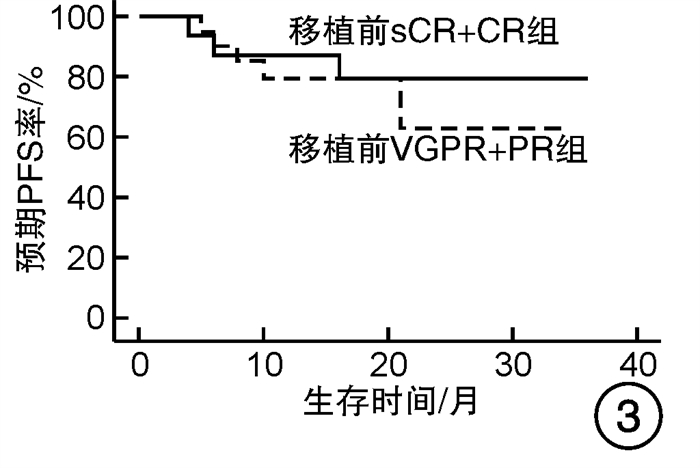
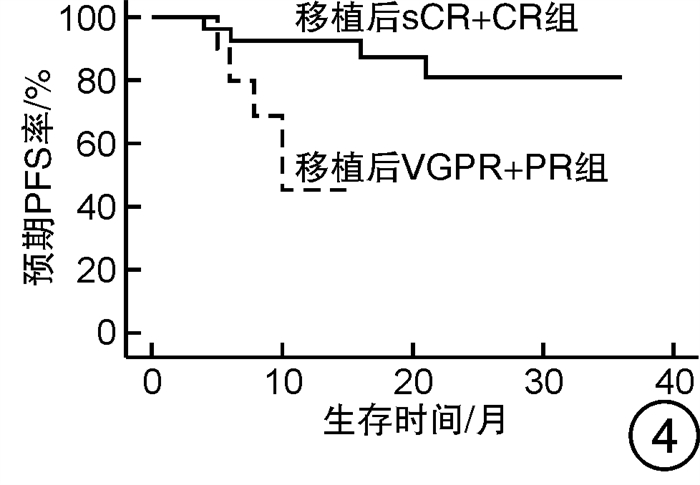
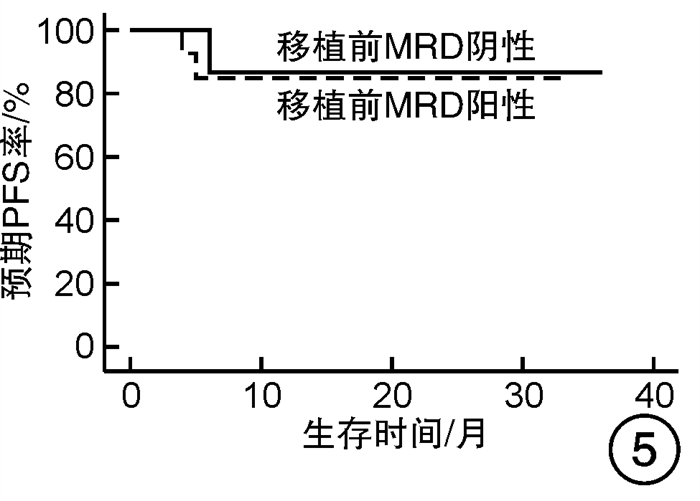
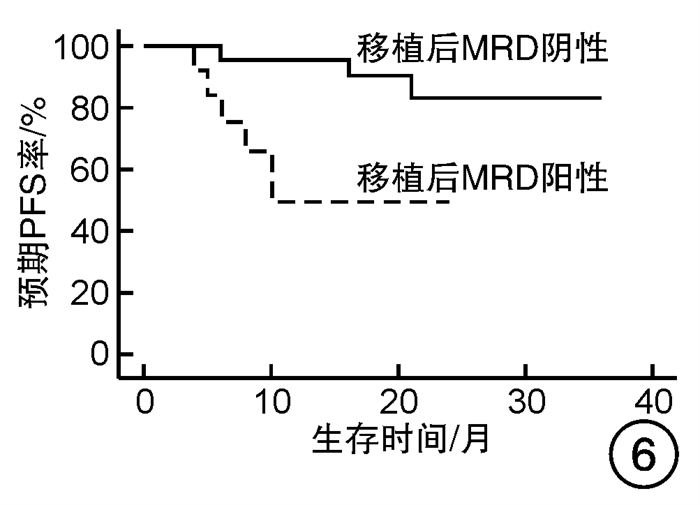
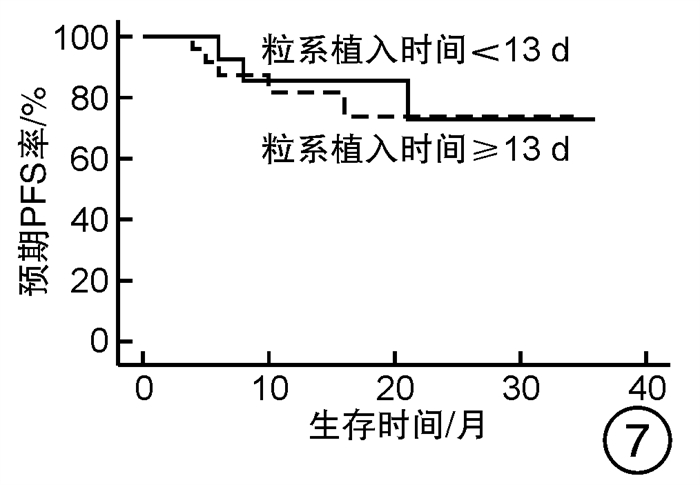
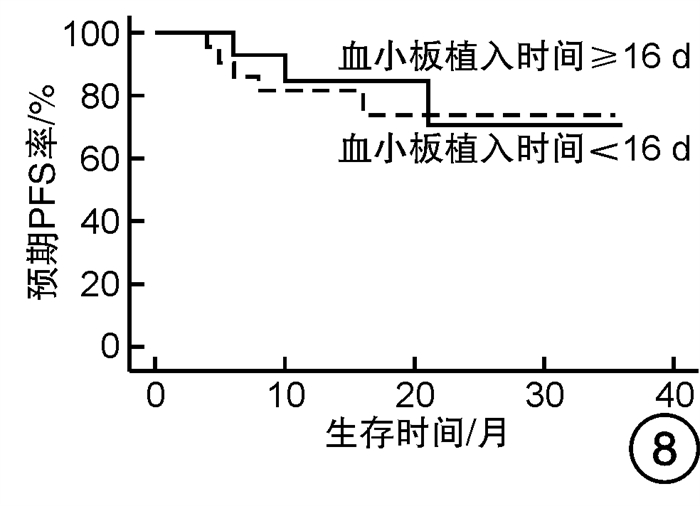
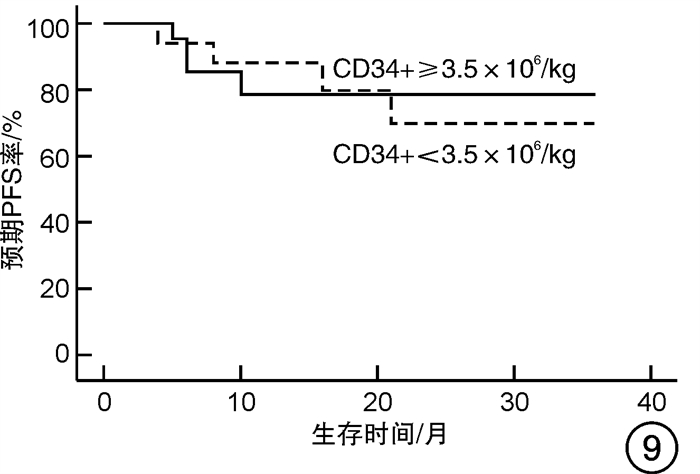
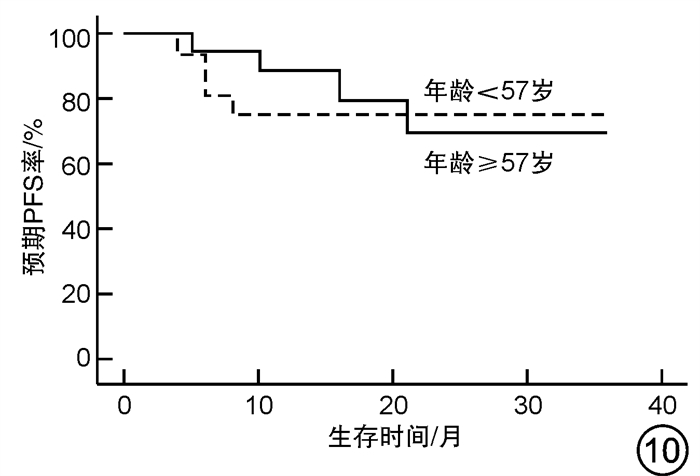
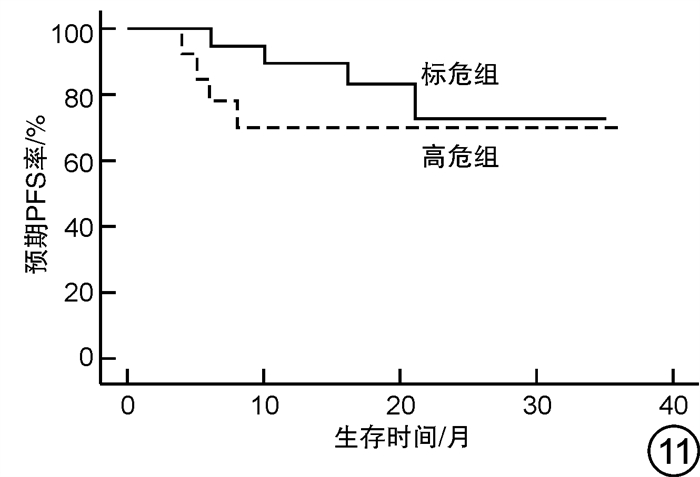
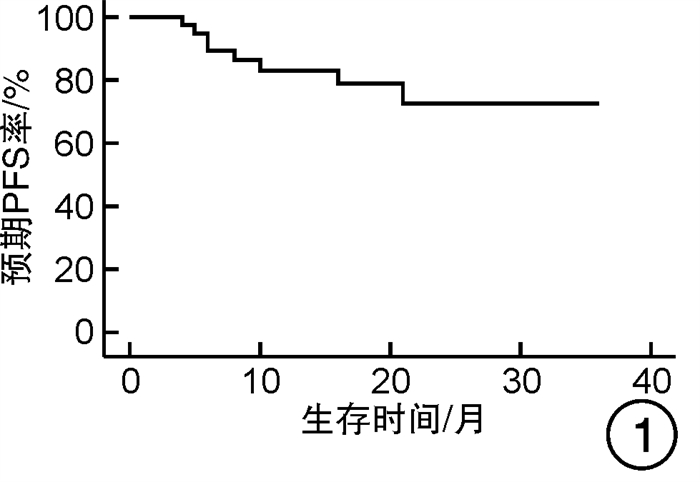
摘要: 目的 对行静脉大剂量美法仑联合外周血自体造血干细胞移植治疗多发性骨髓瘤(multiple myeloma,MM)患者的无进展生存期(progression-free survival,PFS)进行回顾性分析研究。方法 回顾性分析2019年8月—2022年6月接受静脉大剂量无丙二醇美法仑预处理联合外周血自体造血干细胞移植的连续42例初治MM患者的临床资料,评估其临床疗效及PFS。结果 42例MM患者均顺利完成造血重建,无移植相关死亡。截止随访终止日期,纳入研究的所有患者3年预期PFS率为72.7%(95%CI 55.26%~90.14%);3年预期总生存率为88.3%(95%CI 71.05%~105.55%)。将移植后疾病状态分为严格意义的完全缓解(sCR)+完全缓解(CR)组(30例)和非常好的部分缓解(VGPR)+部分缓解(PR)组(12例),sCR+CR组的3年预期PFS率为81.1%(95%CI 63.40%~98.74%),VGPR+PR组的3年预期PFS率为45.7%(95%CI 4.15%~87.25%),差异有统计学意义(P=0.012)。对于行美法仑联合外周血自体造血干细胞移植的MM患者,造血重建时间、CD34+细胞输注数量及中位年龄不是PFS的独立影响因素。自体移植后微小残留病阴性患者的2年预期PFS率为83.1%(95%CI 64.28%~101.92%),微小残留病阳性患者的2年预期PFS率为49.6%(95%CI 14.91%~84.29%),差异有统计学意义(P=0.004)。纳入研究的所有患者中,标危患者3年预期PFS率为73.8%(95%CI 49.50%~98.10%),高危患者3年预期PFS率为71.4%(95%CI 47.68%~95.11%),差异无统计学意义(P=0.318)。结论 静脉无丙二醇美法仑可安全应用于患者的自体外周血造血干细胞。移植后呈sCR+CR的MM患者PFS较VGPR+PR患者的PFS得到显著改善。行自体移植后微小残留病阴性可以使PFS获益。大剂量美法仑联合外周血自体造血干细胞移植能克服mSMART3.0分层高危的不良因素,显著提升MM患者的CR率。Abstract: Objective To retrospectively analyze the progression-free survival(PFS) of patients with multiple myeloma(MM) treated by intravenous high-dose melphalan combined with peripheral blood autologous hematopoietic stem cell transplantation.Methods The clinical data of 42 consecutive patients with newly diagnosed MM who received intravenous high-dose melphalan pretreatment without propanediol combined with autologous peripheral blood stem cell transplantation from August 2019 to June 2022 were analyzed retrospectively, and the clinical efficacy and PFS were evaluated.Results All 42 patients with MM completed hematopoietic reconstitution successfully and there was no transplant-related death. By the end of the follow-up date, the 3-year expected PFS of all patients enrolled in the study was 72.7%(95%CI 55.26%-90.14%), and the 3-year expected overall survival was 88.3%(95%CI 71.05%-105.55%). Patients were divided into sCR+CR group(n=30) and VGPR+PR group(n=12) according to the disease status after transplantation. The 3-year expected PFS of sCR+CR group was 81.1%(95%CI 63.40%-98.74%), and the 3-year expected PFS of VGPR+PR group was 45.7%(95%CI 4.15%-87.25%), there was a significant difference between the 2 groups(P=0.012). For MM patients who received melphalan combined with autologous peripheral blood stem cell transplantation, the hematopoietic reconstitution time, the number of CD34+ cells and the median age were not independent influencing factors of PFS. The 2-year expected PFS of MRD-negative patients after autologous transplantation was 83.1%(95%CI 64.28%-101.92%), and the 2-year expected PFS of MRD-positive patients was 49.6%(95%CI 14.91%-84.29%), there was a significant difference between the 2 groups(P=0.004). Among all the patients included in the study, the 3-year expected PFS of standard risk patients was 73.8%(95%CI 49.50%-98.10%), and that of high-risk patients was 71.4%(95%CI 47.68%-95.11%), there was no significant difference between the 2 groups(P=0.318).Conclusion Intravenous melphalan without propylene glycol can be safely used in patients with autologous peripheral blood stem cells. The PFS of MM patients with sCR+CR after transplantation is significantly better than that of VGPR+PR patients. Negative MRD after autologous transplantation can benefit PFS. High-dose melphalan combined with autologous peripheral blood stem cell transplantation can overcome the high risk factors of mSMART3.0 stratification and significantly increase the rate of CR in patients with MM.
-
Key words:
- multiple myeloma /
- autologous transplantation /
- prognosis /
- intravenous mepharam
-

-
[1] Usmani SZ, Hoering A, Cavo M, et al. Clinical predictors of long-term survival in newly diagnosed transplant eligible multiple myeloma-an IMWG Research Project[J]. Blood Cancer J, 2018, 8(12): 123. doi: 10.1038/s41408-018-0155-7
[2] Mikhael J, Ismaila N, Cheung MC, et al. Treatment of Multiple Myeloma: ASCO and CCO Joint Clinical Practice Guideline[J]. J Clin Oncol, 2019, 37(14): 1228-1263. doi: 10.1200/JCO.18.02096
[3] Dimopoulos MA, Moreau P, Terpos E, et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up[J]. Ann Oncol, 2021, 32(3): 309-322. doi: 10.1016/j.annonc.2020.11.014
[4] Cavo M, Gay F, Beksac M, et al. Autologous haematopoietic stem-cell transplantation versus bortezomib-melphalan-prednisone, with or without bortezomib-lenalidomide-dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma(EMN02/HO95): a multicentre, randomised, open-label, phase 3 study[J]. Lancet Haematol, 2020, 7(6): e456-e468. doi: 10.1016/S2352-3026(20)30099-5
[5] Gay F, Musto P, Rota-Scalabrini D, et al. Carfilzomib with cyclophosphamide and dexamethasone or lenalidomide and dexamethasone plus autologous transplantation or carfilzomib plus lenalidomide and dexamethasone, followed by maintenance with carfilzomib plus lenalidomide or lenalidomide alone for patients with newly diagnosed multiple myeloma(FORTE): a randomised, open-label, phase 2 trial[J]. Lancet Oncol, 2021, 22(12): 1705-1720. doi: 10.1016/S1470-2045(21)00535-0
[6] Attal M, Harousseau JL, Stoppa AM, et al. Randomized Trial of Autologous Bone Marrow Transplantation and Chemotherapy in Multiple Myeloma[J]. N Engl J Med, 1996, 335(2): 91-97. doi: 10.1056/NEJM199607113350204
[7] Stadtmauer EA, Pasquini MC, Blackwell B, et al. Autologous Transplantation, Consolidation, and Maintenance Therapy in Multiple Myeloma: Results of the BMT CTN 0702 Trial[J]. J Clin Oncol, 2019, 37(7): 589-597. doi: 10.1200/JCO.18.00685
[8] Mina R, Gay F. The role of autologous stem-cell transplantation in multiple myeloma in 2021[J]. Curr Opin Oncol, 2021, 33(6): 642-647. doi: 10.1097/CCO.0000000000000783
[9] Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma[J]. Lancet Oncol, 2016, 17(8): e328-e346. doi: 10.1016/S1470-2045(16)30206-6
[10] Gonsalves WI, Buadi FK, Ailawadhi S, et al. Utilization of hematopoietic stem cell transplantation for the treatment of multiple myeloma: a Mayo Stratification of Myeloma and Risk-Adapted Therapy(mSMART)consensus statement[J]. Bone Marrow Transplant, 2019, 54(3): 353-367. doi: 10.1038/s41409-018-0264-8
[11] Parrondo RD, Ailawadhi S, Sher T, et al. Autologous Stem-Cell Transplantation for Multiple Myeloma in the Era of Novel Therapies[J]. JCO Oncol Pract, 2020, 16(2): 56-66. doi: 10.1200/JOP.19.00335
[12] 李娟, 刘俊茹. 自体造血干细胞移植在多发性骨髓瘤治疗中的优化和生存趋势[J]. 临床血液学杂志, 2021, 34(7): 449-453. doi: 10.13201/j.issn.1004-2806.2021.07.001 https://lcxy.whuhzzs.com/article/doi/10.13201/j.issn.1004-2806.2021.07.001
[13] 梁惠如, 邹茂权, 萧杏贤, 等. 自体造血干细胞移植治疗不同年龄段多发性骨髓瘤的安全性和有效性分析[J]. 临床血液学杂志, 2019, 32(9): 693-696. doi: 10.13201/j.issn.1004-2806.2019.09.011
[14] Devarakonda S, Efebera Y, Sharma N. Role of Stem Cell Transplantation in Multiple Myeloma[J]. Cancers(Basel), 2021, 13(4): 863.
[15] Zar T, Graeber C, Perazella MA, et al. Treatment, and Prevention of Propylene Glycol Toxicity[J]. Semin Dial, 2007, 20(3): 217-219. doi: 10.1111/j.1525-139X.2007.00280.x
[16] Singh R, Chen J, Miller T, et al. Solution stability of Captisol-stabilized melphalan(Evomela)versus Propylene glycol-based melphalan hydrochloride injection[J]. Pharm Dev Technol, 2018, 23(10): 1024-1029. doi: 10.1080/10837450.2016.1265557
[17] Kumar SK, Callander NS, Adekola K, et al. Multiple Myeloma, Version 3.2021, NCCN Clinical Practice Guidelines in Oncology[J]. J Natl Compr Canc Netw, 2020, 18(12): 1685-1717. doi: 10.6004/jnccn.2020.0057
[18] Kazandjian D, Dew A, Hill E. The changing role of high dose melphalan with stem cell rescue in the treatment of newly diagnosed multiple myeloma in the era of modern therapies-back to the future![J]. Best Pract Res Clin Haemato, 2020, 33(1): 101150. doi: 10.1016/j.beha.2020.101150
[19] Stadtmauer EA, Pasquini MC, Blackwell B, et al. Autologous Transplantation, Consolidation, and Maintenance Therapy in Multiple Myeloma: Results of the BMT CTN 0702 Trial[J]. J Clin Oncol, 2019, 37(7): 589-597. doi: 10.1200/JCO.18.00685
[20] Tacchetti P, Pantani L, Patriarca F, et al. Bortezomib, thalidomide, and dexamethasone followed by double autologous haematopoietic stem-cell transplantation for newly diagnosed multiple myeloma(GIMEMA-MMY-3006): long-term follow-up analysis of a randomised phase 3, open-label study[J]. Lancet Haematol, 2020, 7(12): e861-e873. doi: 10.1016/S2352-3026(20)30323-9
[21] 隋伟薇, 邹德慧, 安刚, 等. 多发性骨髓瘤患者自体造血干细胞移植后长期随访的单中心结果[J]. 中华血液学杂志, 2017, 38(6): 499-504.
[22] Munshi NC, Avet-Loiseau H, Anderson KC, et al. A large meta-analysis establishes the role of MRD negativity in long-term survival outcomes in patients with multiple myeloma[J]. Blood Adv, 2020, 4(23): 5988-5999. doi: 10.1182/bloodadvances.2020002827
[23] Avet-Loiseau H, Ludwig H, Landgren O, et al. Minimal Residual Disease Status as a Surrogate Endpoint for Progression-free Survival in Newly Diagnosed Multiple Myeloma Studies: A Meta-analysis[J]. Clin Lymphoma Myeloma Leuk, 2020, 20(1): e30-e37. doi: 10.1016/j.clml.2019.09.622
[24] Nunnelee J, Cottini F, Zhao Q, et al. Improvement in Post-Autologous Stem Cell Transplant Survival of Multiple Myeloma Patients: A Long-Term Institutional Experience[J]. Cancers(Basel), 2022, 14(9): 2277.
[25] Hari P, Aljitawi OS, Arce-Lara C, et al. A Phase Ⅱb, Multicenter, Open-Label, Safety, and Efficacy Study of High-Dose, Propylene Glycol-Free Melphalan Hydrochloride for Injection(EVOMELA)for Myeloablative Conditioning in Multiple Myeloma Patients Undergoing Autologous Transplantation[J]. Biol Blood Marrow Transplant, 2015, 21(12): 2100-2105. doi: 10.1016/j.bbmt.2015.08.026
-




 下载:
下载: