Efficacy and safety of hetrombopag in the treatment of chronic primary immune thrombocytopenia
-
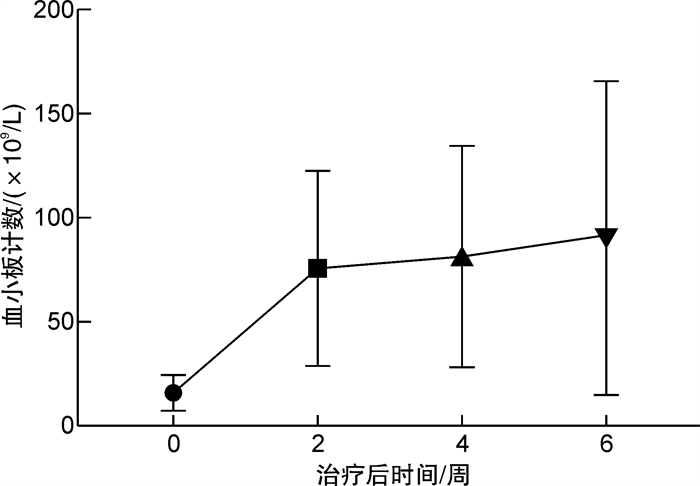
摘要: 目的 探究海曲泊帕治疗慢性原发免疫性血小板减少症(primary immune thrombocytopenia,ITP)的临床疗效和安全性。方法 选取2021年6月—2022年12月我院血液内科收治的25例慢性ITP患者纳入本研究,观察海曲泊帕治疗慢性ITP的有效率、起效时间、达到峰值所需时间、治疗前后血小板水平以及不良反应。结果 本研究中海曲泊帕治疗慢性ITP患者的疗效显著,总有效率达80%;中位起效时间为12(5~23) d;达到血小板峰值所需的中位时间为4.5(1.5~6.5)周。25例慢性ITP患者治疗前血小板平均水平为(13.71±5.31)×109/L,治疗后显著上升至(83.35±6.28)×109/L,差异有统计学意义(P < 0.05)。25例慢性ITP患者治疗前出血评分为0、1、2级者分别为6、15、4例,治疗后分别为17、5、3例。治疗后出血评分显著降低,与治疗前比较差异有统计学意义(P < 0.05)。治疗期间出现肝功能异常患者3例,予以口服保肝药物治疗后肝功能均恢复正常;出现腹泻患者1例,予以口服止泻药物后好转;无一例患者因不良反应停药。结论 海曲泊帕治疗慢性ITP患者的疗效显著,安全性良好,可作为慢性ITP患者的治疗方案之一。
-
关键词:
- 慢性免疫性血小板减少症 /
- 海曲泊帕 /
- 疗效 /
- 安全性
Abstract: Objective To investigate the clinical efficacy and safety of hetrombopag in the treatment of chronic primary immune thrombocytopenia(ITP).Methods A total of 25 patients with chronic ITP were enrolled from June 2021 to December 2022. The effective rate, onset time, peak time, platelet level before and after treatment and adverse events of hetrombopag in the treatment of chronic ITP were observed.Results The results of this study showed that hetrombopag was effective in the treatment of patients with chronic ITP, and the total effective rate was 80%, the median effective time was 12(5-23) days, and the median time to reach platelet peak was 4.5(1.5-6.5) weeks. The average platelet level of 25 patients with chronic ITP before treatment was (13.71±5.31) ×109/L. It was significantly increased to (83.35±6.28)×109/L after treatment(P < 0.05). Before treatment, the bleeding scores of grade 0, 1 and 2 in 25 patients were 6 cases, 15 cases and 4 cases, respectively, and after treatment there were 17 cases, 5 cases and 3 cases, respectively. The bleeding score decreased significantly after treatment(P < 0.05). During the treatment, there were 3 cases of abnormal liver function, which returned to normal after oral treatment with hepatoprotective drugs, and 1 case of diarrhea improved after oral antidiarrheal drugs, and no patient stopped taking drugs because of adverse events.Conclusion Hetrombopag is effective and safe in the treatment of chronic ITP patients, and can be used as one of the treatment options for chronic ITP patients.-
Key words:
- chronic primary immune thrombocytopenia /
- hetrombopag /
- efficacy /
- safety
-

-
[1] Bussel J, Cooper N, Boccia R, et al. Immune thrombocytopenia[J]. Expert Rev Hematol, 2021, 14(11): 1013-1025. doi: 10.1080/17474086.2021.1995347
[2] Rodriguez-Vigil Iturrate C, Sanz De Miguel MP, Martinez Faci C, et al. Primary immune thrombocytopenia: Experience of a specialised clinic[J]. An Pediatr(Engl Ed), 2020, 93(1): 16-23.
[3] 桑海强, 马慧慧, 冯蕊涵. 替罗非班诱导的血小板减少症对患者预后影响的回顾性对照研究[J]. 临床心血管病杂志, 2022, 38(2): 113-118. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXB202202007.htm
[4] 郭世杰, 齐向前. 糖皮质激素和(或)免疫球蛋白治疗替罗非班诱导重度血小板减少症的临床观察[J]. 临床心血管病杂志, 2021, 37(5): 417-420. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXB202105006.htm
[5] Visweshwar N, Ayala I, Jaglal M, et al. Primary immune thrombocytopenia: a 'diagnosis of exclusion'?[J]. Blood Coagul Fibrinolysis, 2022, 33(6): 289-294. doi: 10.1097/MBC.0000000000001144
[6] Mingot-Castellano ME. New treatments for primary immune thrombocytopenia[J]. Blood Coagul Fibrinolysis, 2022, 33(Suppl 1): S8-S11.
[7] 曹璇, 杨仁池. 慢性成人原发免疫性血小板减少症的治疗进展[J]. 临床血液学杂志, 2021, 34(1): 76-80. https://lcxy.whuhzzs.com/article/doi/10.13201/j.issn.1004-2806.2021.01.017
[8] Wang Y, Sheng L, Han F, et al. Efficacy and safety of treatments in newly diagnosed adult primary immune thrombocytopenia: A systematic review and network meta-analysis[J]. EClinicalMedicine, 2023, 56: 101777. doi: 10.1016/j.eclinm.2022.101777
[9] Lozano ML. New developments in the diagnosis of primary immune thrombocytopenia[J]. Blood Coagul Fibrinolysis, 2022, 33(Suppl 1): S5-S7.
[10] 中华医学会血液学分会血栓与止血学组. 成人原发免疫性血小板减少症诊断与治疗中国指南(2020年版)[J]. 中华血液学杂志, 2020, 41(8): 617-623. https://www.cnki.com.cn/Article/CJFDTOTAL-LCLZ202106026.htm
[11] Ebbo M, Riviere E, Godeau B. Adult immune thrombocytopenia and thrombopoietin receptor agonist: Ten years later[J]. Rev Med Interne, 2021, 42(1): 38-45. doi: 10.1016/j.revmed.2020.05.017
[12] 侯明, 刘新光. 立足中国实际的原发免疫性血小板减少症诊治——2020版成人原发免疫性血小板减少症诊断与治疗中国指南解读[J]. 临床血液学杂志, 2021, 34(1): 1-4. https://lcxy.whuhzzs.com/article/doi/10.13201/j.issn.1004-2806.2021.01.001
[13] Yu Y, Hou Y, Zhao Y, et al. Platelet autoantibody specificity and response to rhTPO treatment in patients with primary immune thrombocytopenia[J]. Br J Haematol, 2021, 194(1): 191-194. doi: 10.1111/bjh.17510
[14] Zhang Y, Kolesar JM. Eltrombopag: an oral thrombopoietin receptor agonist for the treatment of idiopathic thrombocytopenic purpura[J]. Clin Ther, 2011, 33(11): 1560-1576. doi: 10.1016/j.clinthera.2011.10.004
[15] Neunert CE. Thrombopoietin Receptor Agonist Use for Immune Thrombocytopaenia[J]. Hamostaseologie, 2019, 39(3): 272-278.
[16] Liu X, Bai Y, Wang T, et al. Recombinant human thrombopoietin(rhTPO)of different dosing regimens for refractory/relapsed primary immune thrombocytopenia: a multicenter, randomized controlled trial and pharmacokinetics study[J]. Platelets, 2023, 34(1): 2157806.
[17] Khalafallah A, Rahman Z, Ogden K, et al. Successful treatment with thrombopoietin receptor agonist in avoiding splenectomy for patients with chronic refractory immune thrombocytopenia[J]. Mediterr J Hematol Infect Dis, 2012, 4(1): e2012003.
[18] Kapur R, Aslam R, Speck ER, et al. Thrombopoietin receptor agonist(TPO-RA)treatment raises platelet counts and reduces anti-platelet antibody levels in mice with immune thrombocytopenia(ITP)[J]. Platelets, 2020, 31(3): 399-402.
[19] Xie C, Zhao H, Bao X, et al. Pharmacological characterization of hetrombopag, a novel orally active human thrombopoietin receptor agonist[J]. J Cell Mol Med, 2018, 22(11): 5367-5377.
[20] Mei H, Liu X, Li Y, et al. A multicenter, randomized phase Ⅲ trial of hetrombopag: a novel thrombopoietin receptor agonist for the treatment of immune thrombocytopenia[J]. J Hematol Oncol, 2021, 14(1): 37.
[21] Mei H, Chen X, Zhou J, et al. Safety and efficacy of hetrombopag in patients with chronic immune thrombocytopenia: a single-arm, open-label, multi-center phase 1 study[J]. Ann Transl Med, 2022, 10(2): 30.
[22] 中国临床肿瘤学会(CSCO)抗肿瘤药物治疗安全管理专家委员会. 海曲泊帕临床应用指导原则[J]. 白血病·淋巴瘤, 2022, 31(10): 577-582.
[23] Syed YY. Hetrombopag: First Approval[J]. Drugs, 2021, 81(13): 1581-1585.
[24] Yang W, Zhao X, Liu X, et al. Hetrombopag plus porcine ATG and cyclosporine for the treatment of aplastic anaemia: early outcomes of a prospective pilot study[J]. Exp Hematol Oncol, 2023, 12(1): 16.
[25] Mei H, Liu X, Li Y, et al. Dose tapering to withdrawal stage and long-term efficacy and safety of hetrombopag for the treatment of immune thrombocytopenia: Results from an open-label extension study[J]. J Thromb Haemost, 2022, 20(3): 716-728.
[26] Abbasi AM, Shaikh MU, Ali N, et al. Response of Eltrombopag in immune thrombocytopenia and acquired idiopathic aplastic anemia: A single-center experience[J]. Leuk Res Rep, 2022, 17: 100295.
-




 下载:
下载: