Efficacy and safety of isavuconazole diagnostic-driven therapy of hematologic malignancies combined with invasive aspergillus pneumonia
-
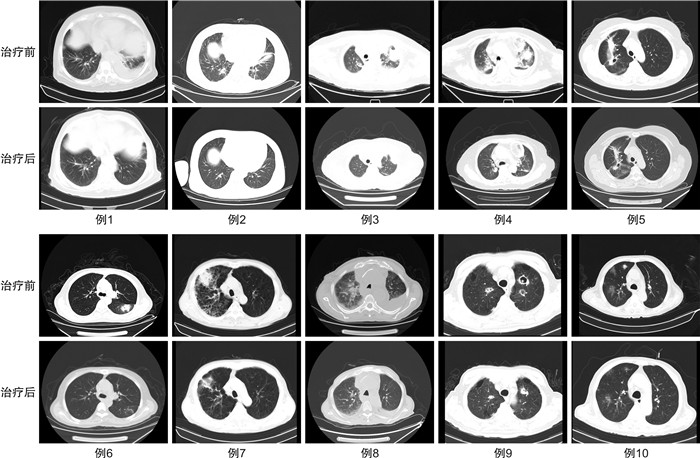
摘要: 目的 评价艾沙康唑胶囊诊断驱动治疗中国恶性血液病患者合并侵袭性曲霉菌肺炎的疗效和安全性。方法 收集青岛大学附属医院2022年6月至2023年9月收治的28例恶性血液病患者,广谱抗生素及抗真菌治疗后仍出现发热且肺部CT表现或真菌抗原检测提示侵袭性曲霉菌肺炎,分析患者行艾沙康唑胶囊单药抗真菌的有效性及安全性。将28例患者分为4组,A组(5例):口服泊沙康唑预防真菌突破;B组(15例):静脉伏立康唑不耐受或无效;C组(5例):可能合并侵袭性毛霉菌感染;D组(3例):静脉卡泊芬净或两性霉素B不耐受或无效。结果 28例患者均为免疫缺陷状态,其中急性白血病占60.71%,基线中性粒细胞计数为0.49(0.24,5.52)×109/L。治疗周期平均为14 d,治疗后总有效率为82.14%(23/28)。3例患者治疗后仍间断发热且肺部CT影像学未见明显改善,1例患者因疾病进展死亡,1例患者治疗中自动出院。安全性分析:所有患者治疗前后的谷草转氨酶、谷丙转氨酶、肌酐、Q-T间期比较差异均无统计学意义(P>0.05),其中B组1例患者换用艾沙康唑治疗后谷草转氨酶、谷丙转氨酶降至正常,2例患者治疗后肌酐未再出现升高,2例患者出现轻度恶心,1例患者出现腹泻;D组1例患者出现转氨酶轻度升高,无患者因药物不良反应而停药。结论 恶性血液病患者因免疫缺陷导致侵袭性霉菌的发病率增加,肺部CT可以提供重要的证据,艾沙康唑胶囊在诊断驱动阶段单药治疗泊沙康唑预防失败及对伏立康唑不耐受/疗效不佳的侵袭性曲霉菌肺炎的有效率高,且艾沙康唑胶囊具有良好的安全性。Abstract: Objective To evaluate the efficacy and safety of isavuconazole capsules in diagnostic-driven treatment of hematologic malignancies with invasive aspergillus pneumonia in Chinese patients.Methods The antifungal efficacy and safety of isavuconazole capsules as monotherapy were retrospectively analyzed from June 2022 to September 2023 in 28 patients with hematologic malignancies, who still had fever after broad-spectrum antibiotics and considered invasive aspergillus pneumonia with lung CT or fungal antigen detection. Patients were divided into four groups, group A(5 cases): invasive fungal infections under posaconazole prevention, group B(15 cases): voriconazole intolerance/ineffectiveness group, group C(5 cases): possible invasive mucormycosis group, group D(3 cases): amphotericin B or caspofungin intolerance/ineffectiveness group.Results All the 28 patients(60.71% with acute leukemia) were immunodeficient. The baseline neutrophil count was 0.49(0.24, 5.52) ×109/L, and the average treatment duration was 14 days. The total effective rate after treatment was 82.14%(23/28). Three patients still had intermittent fever and no imaging improvement after treatment, 1 patient died after disease progression, 1 patient was discharged automatically during treatment. There were no significant differences in aspartate aminotransferase, alanine aminotransferase, creatinine and Q-T interval among the four groups before and after treatment(P>0.05), among which 1 patient in group B had aspartate aminotransferase and alanine aminotransferase reduced to normal after isavuconazole treatment, creatinine did not rise again in 2 patients after treatment, nausea occurred in 2 patients, diarrhea occurred in 1 patient. One patient in group D had aspartate aminotransferase and alanine aminotransferase elevated slightly and no patient stopped taking the drug due to adverse events.Conclusion Immune deficiencies enhance the likelihood of invasive fungal disease in patients with malignant hematologic diseases. Lung CT can provide significant evidence. Isavuconazole capsules monotherapy is highly effective and safety in failure of posaconazole prevention or voriconazole intolerance/ineffectiveness during the diagnostic driving stage of aspergillosis pneumonia.
-
Key words:
- isavuconazole /
- invasive aspergillosis /
- hematologic malignancies /
- diagnostic-driven therapy /
- security
-

-
[1] Sun Y, Huang H, Chen J, et al. Invasive fungal infection in patients receiving chemotherapy for hematological malignancy: a multicenter, prospective, observational study in China[J]. Tumour Biol, 2015, 36(2): 757-67. doi: 10.1007/s13277-014-2649-7
[2] 中国医师协会血液科医师分会, 中国侵袭性真菌感染工作组. 血液病/恶性肿瘤患者侵袭性真菌病的诊断标准与治疗原则(第六次修订版)[J]. 中华内科杂志, 2020, 59(10): 754-763. https://www.cnki.com.cn/Article/CJFDTOTAL-YLYS201720099.htm
[3] Ananda-Rajah MR, Kontoyiannis D. Isavuconazole: a new extended spectrum triazole for invasive mold diseases[J]. Future Microbiol, 2015, 10(5): 693-708. doi: 10.2217/fmb.15.34
[4] 张婷婷, 孙玲洁, 冯四洲. 艾沙康唑治疗侵袭性真菌病的临床研究进展[J]. 中国感染与化疗杂志, 2022, 22(3): 360-365. doi: 10.16718/j.1009-7708.2022.03.023
[5] 中华医学会血液学分会抗感染学组. 艾沙康唑临床应用专家共识(2023版)[J]. 临床血液学杂志, 2023, 36(5): 295-302. doi: 10.13201/j.issn.1004-2806.2023.05.001
[6] 杨政, 刘正印. 毛霉菌病的诊断和治疗进展[J]. 中华内科杂志, 2021, 60(11): 1013-1016.
[7] Zhang T, Shen Y, Feng S. Clinical research advances of isavuconazole in the treatment of invasive fungal diseases[J]. Front Cell Infect Microbiol, 2022, 12: 1049959. doi: 10.3389/fcimb.2022.1049959
[8] Fracchiolla NS, Sciumè M, Orofino N, et al. Epidemiology and treatment approaches in management of invasive fungal infections in hematological malignancies: Results from a single-centre study[J]. PLos One, 2019, 14(5): e0216715. doi: 10.1371/journal.pone.0216715
[9] 胡炯. 对血液系统疾病及其肿瘤患者的抗真菌经验和诊断驱动治疗策略[J]. 上海医药, 2014, 35(9): 11-14. https://www.cnki.com.cn/Article/CJFDTOTAL-SYIY201409004.htm
[10] Houst J, Spizek J, Havlicek V. Antifungal Drugs[J]. Metabolites, 2020, 10(3): 106. doi: 10.3390/metabo10030106
[11] Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management[J]. Eur Respir J, 2016, 47(1): 45-68. doi: 10.1183/13993003.00583-2015
[12] Roth RS, Masouridi-Levrat S, Giannotti F, et al. Frequency and causes of antifungal treatment changes in allogeneic haematopoietic cell transplant recipients with invasive mould infections[J]. Mycoses, 2022, 65(2): 199-210. doi: 10.1111/myc.13416
[13] Czyrski A, Resztak M, Swiderski P, et al. The Overview on the Pharmacokinetic and Pharmacodynamic Interactions of Triazoles[J]. Pharmaceutics, 2021, 13(11): 1961. doi: 10.3390/pharmaceutics13111961
[14] Girmenia C, Iori AP. An update on the safety and interactions of antifungal drugs in stem cell transplant recipients[J]. Expert Opin Drug Saf, 2017, 16(3): 329-339. doi: 10.1080/14740338.2017.1273900
[15] Ledoux MP, Denis J, Nivoix Y, et al. Isavuconazole: A new broad-spectrum azole. Part 2: pharmacokinetics and clinical activity[J]. J Mycol Med, 2018, 28(1): 15-22. doi: 10.1016/j.mycmed.2018.02.002
[16] McCreary EK, Nguyen MH, Davis MR, et al. Achievement of clinical isavuconazole blood concentrations in transplant recipients with isavuconazonium sulphate capsules administered via enteral feeding tube[J]. J Antimicrob Chemother, 2020, 75(10): 3023-3028. doi: 10.1093/jac/dkaa274
[17] Maertens JA, Raad II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi(SECURE): a phase 3, randomised-controlled, non-inferiority trial[J]. Lance, 2016, 387(10020): 760-769. doi: 10.1016/S0140-6736(15)01159-9
[18] Shirley M, Scott LJ. Isavuconazole: A Review in Invasive Aspergillosis and Mucormycosis[J]. Drugs, 2016, 76(17): 1647-1657. doi: 10.1007/s40265-016-0652-6
[19] Townsend RW, Akhtar S, Alcorn H, et al. Phase Ⅰ trial to investigate the effect of renal impairment on isavuconazole pharmacokinetics[J]. Eur J Clin Pharmacol, 2017, 73(6): 669-678. doi: 10.1007/s00228-017-2213-7
[20] Bose P, McCue D, Wurster S, et al. Isavuconazole as Primary Antifungal Prophylaxis in Patients With Acute Myeloid Leukemia or Myelodysplastic Syndrome: An Open-label, Prospective, Phase 2 Study[J]. Clin Infect Dis, 2021, 72(10): 1755-1763.
[21] Scott SA, Perry C, Mahmoudjafari Z, et al. Incidence of breakthrough fungal infections on isavuconazole prophylaxis compared to posaconazole and voriconazole[J]. Transpl Infect Dis, 2023, 25(2): e14045. doi: 10.1111/tid.14045
[22] Dagher H, Hachem R, Chaftari AM, et al. Real-World Use of Isavuconazole as Primary Therapy for Invasive Fungal Infections in High-Risk Patients with Hematologic Malignancy or Stem Cell Transplant[J]. J Fungi(Basel), 2022, 8(1): 74.
[23] DiPippo AJ, Rausch CR, Kontoyiannis DP. Tolerability of isavuconazole after posaconazole toxicity in leukaemia patients[J]. Mycoses, 2019, 62(1): 81-86. doi: 10.1111/myc.12851
-




 下载:
下载: