Potential mechanism of lactate dehydrogenase A acetylation regulation to promote bortezomib resistance in multiple myeloma
-
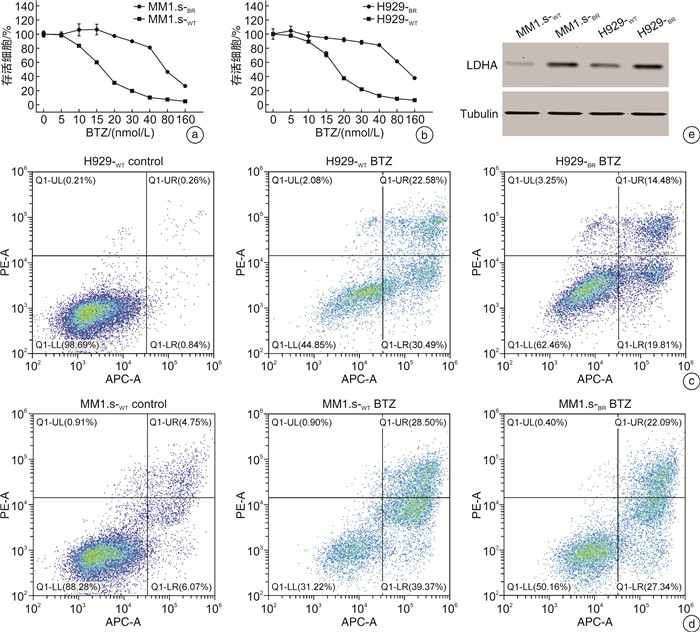
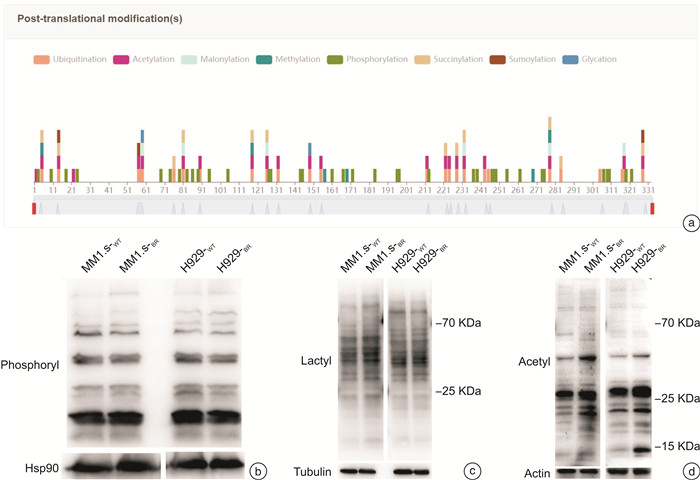
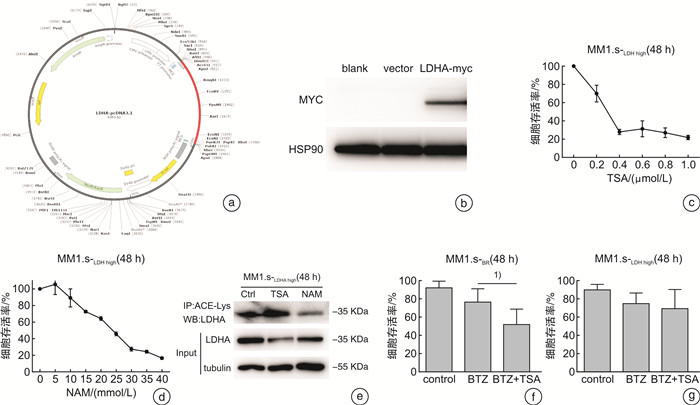
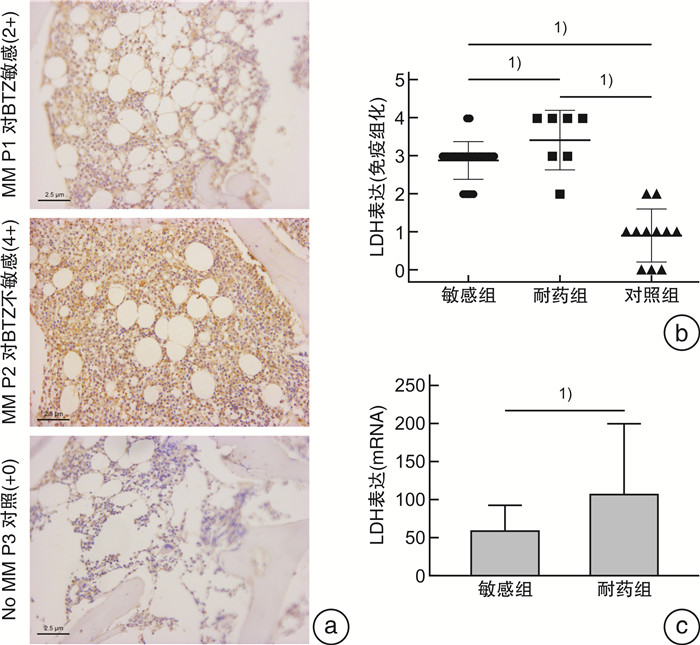
摘要: 目的 探讨乳酸脱氢酶A(lactate dehydrogenase A,LDHA)乙酰化调节促进多发性骨髓瘤(multiple myeloma,MM)对硼替佐米(bortezomib,BTZ)耐药的可能机制。方法 在骨髓瘤患者中检测LDHA表达与BTZ敏感性的关系。构建对BTZ耐药的骨髓瘤细胞株,检测耐药株与敏感株LDHA的表达情况。用CCK8检测BTZ处理后骨髓瘤耐药和敏感株的细胞增殖,用流式细胞仪检测BTZ处理后耐药和敏感株的凋亡率。在iHypoxia数据库中对LDHA可能的翻译后修饰位点进行预测,并对常见的翻译后修饰进行检测。为了探索LDHA上游调控机制,使用不同的抑制剂处理骨髓瘤细胞后检测LDHA的表达情况。结果 MM患者肿瘤组织的LDHA表达明显高于对照组,且对BTZ耐药患者LDHA的蛋白和mRNA表达明显高于对BTZ敏感的患者。成功构建BTZ诱导耐药的MM1.s-BR和NCI-H929-BR耐药细胞株。CCK8检测细胞活性证明BTZ耐药细胞株在BTZ处理后存活率高于敏感细胞株,而流式细胞仪结果显示BTZ敏感细胞株在BTZ处理后凋亡率高于耐药细胞株,以上结果证实BTZ耐药细胞株的构建成功。Western blot结果显示LDHA在BTZ耐药细胞株中的表达高于野生型细胞株。通过数据库预测,LDHA常见的翻译后修饰位点为磷酸化、乙酰化和泛素化。BTZ耐药细胞株中乙酰化水平较敏感株明显下降,而其他翻译后修饰程度耐药株和敏感株无明显差异。组蛋白脱乙酰酶类Ⅰ和Ⅱ的共同抑制剂trichostatin处理BTZ耐药细胞株后LDHA蛋白含量下降,乙酰化水平上调,且可以克服耐药株MM1.s-BR对BTZ的耐药,但不能克服高表达LDHA的MM1.s LDH high对BTZ的耐药。结论 组蛋白脱乙酰酶类Ⅰ/Ⅱ可能通过促进LDHA的去乙酰化提高LDHA水平,促进MM对BTZ耐药。Abstract: Objective To explore the potential mechanism by which the acetylation regulation of lactate dehydrogenase A(LDHA) promotes bortezomib(BTZ) resistance in multiple myeloma(MM).Methods The relationship between LDHA expression and bortezomib sensitivity was examined in myeloma patients. Myeloma cell lines resistant to BTZ were constructed to detect the expression of LDHA in resistant and sensitive strains. The cell proliferation of resistant and sensitive myeloma strains after BTZ treatment was detected by CCK8, and the apoptosis rate of resistant and sensitive myeloma strains after BTZ treatment was examined by flow cytometry. Potential post-translational modification sites for LDHA were predicted using the iHypoxia database, and common post-translational modifications were tested. In order to explore the upstream regulation mechanism of LDHA, the expression of LDHA was detected after treating myeloma cells with different inhibitors.Results The expression of LDHA in tumor tissues of MM patients was significantly higher than that of control, and both the level of LDHA protein and mRNA in BTZ-resistant patients were significantly higher than that in BTZ-sensitive patients. MM1.s-BR and NCI-H929-BR resistant cell lines induced by BTZ were successfully constructed. CCK8 showed that the survival rate of BTZ-resistant cell lines after BTZ treatment was higher than that of sensitive cell lines, while flow cytometry results showed that the apoptosis rate of BTZ-sensitive cell lines was higher than that of drug-resistant cell lines after treated with BTZ. The above results confirmed the successful construction of BTZ-resistant cell lines. Western blot results showed that the expression of LDHA in BTZ resistant cell lines was higher than that in wild-type cell lines. As predicted by the database, the common post-translational modification sites of LDHA were phosphorylation, acetylation and ubiquitination. The acetylation level of BTZ resistant cell lines was significantly lower than that of sensitive cell lines, but there was no significant difference in the degree of other post-translational modification between resistant and sensitive cell lines. Administration of trichostatin(TSA), a common inhibitor of histone deacetylase(HDAC) Ⅰand Ⅱ, could decrease the level of LDHA and increase its acetylation level in BTZ resistant cell lines. TSA could overcome the resistance of MM1.s-BRto BTZ, but not the BTZ resistance of MM1.s LDH high BRwith high expression of LDHA.Conclusion HDACⅠ/Ⅱmay increase the level of LDHA by promoting its deacetylation and contribute to the resistance of MM to BTZ.
-

-
[1] van de Donk N, Pawlyn C, Yong KL. Multiple myeloma[J]. Lancet, 2021, 397(10272): 410-427. doi: 10.1016/S0140-6736(21)00135-5
[2] Granja S, Pinheiro C, Reis RM, et al. Glucose Addiction in Cancer Therapy: Advances and Drawbacks[J]. Curr Drug Metab, 2015, 16(3): 221-242. doi: 10.2174/1389200216666150602145145
[3] El AC, De Veirman K, Maes K, et al. Metabolic Features of Multiple Myeloma[J]. Int J Mol Sci, 2018, 19(4): 1200. doi: 10.3390/ijms19041200
[4] Weir P, Donaldson D, Mcmullin MF, et al. Metabolic Alterations in Multiple Myeloma: From Oncogenesis to Proteasome Inhibitor Resistance[J]. Cancers(Basel), 2023, 15(6): 1682.
[5] Augoff K, Hryniewicz-Jankowska A, Tabola R. Lactate dehydrogenase 5: an old friend and a new hope in the war on cancer[J]. Cancer Lett, 2015, 358(1): 1-7. doi: 10.1016/j.canlet.2014.12.035
[6] Huang B, Lu J, Wang X, et al. Prognostic value of lactate dehydrogenase in Chinese patients with newly diagnosed transplant eligible multiple myeloma[J]. Leuk Lymphoma, 2017, 58(7): 1740-1742. doi: 10.1080/10428194.2016.1252975
[7] Lin Y, Wang Y, Li PF. Mutual regulation of lactate dehydrogenase and redox robustness[J]. Front Physiol, 2022, 13: 1038421. doi: 10.3389/fphys.2022.1038421
[8] Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma[J]. Lancet Oncol, 2014, 15(12): e538-e548. doi: 10.1016/S1470-2045(14)70442-5
[9] Sharma D, Singh M, Rani R. Role of LDH in tumor glycolysis: Regulation of LDHA by small molecules for cancer therapeutics[J]. Semin Cancer Biol, 2022, 87: 184-195. doi: 10.1016/j.semcancer.2022.11.007
[10] Feng Y, Xiong Y, Qiao T, et al. Lactate dehydrogenase A: A key player in carcinogenesis and potential target in cancer therapy[J]. Cancer Med, 2018, 7(12): 6124-6136. doi: 10.1002/cam4.1820
[11] Valvona CJ, Fillmore HL, Nunn PB, et al. The Regulation and Function of Lactate Dehydrogenase A: Therapeutic Potential in Brain Tumor[J]. Brain Pathol, 2016, 26(1): 3-17. doi: 10.1111/bpa.12299
[12] Cai H, Li J, Zhang Y, et al. LDHA Promotes Oral Squamous Cell Carcinoma Progression Through Facilitating Glycolysis and Epithelial-Mesenchymal Transition[J]. Front Oncol, 2019, 9: 1446. doi: 10.3389/fonc.2019.01446
[13] Lv J, Zhou Z, Wang J, et al. Prognostic Value of Lactate Dehydrogenase Expression in Different Cancers: A Meta-Analysis[J]. Am J Med Sci, 2019, 358(6): 412-421. doi: 10.1016/j.amjms.2019.09.012
[14] Choudhary C, Kumar C, Gnad F, et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions[J]. Science, 2009, 325(5942): 834-840. doi: 10.1126/science.1175371
[15] He TL, Zhang YJ, Jiang H, et al. The c-Myc-LDHA axis positively regulates aerobic glycolysis and promotes tumor progression in pancreatic cancer[J]. Med Oncol, 2015, 32(7): 187. doi: 10.1007/s12032-015-0633-8
[16] Maiso P, Huynh D, Moschetta M, et al. Metabolic signature identifies novel targets for drug resistance in multiple myeloma[J]. Cancer Res, 2015, 75(10): 2071-2082. doi: 10.1158/0008-5472.CAN-14-3400
[17] Jin L, Chun J, Pan C, et al. Phosphorylation-mediated activation of LDHA promotes cancer cell invasion and tumour metastasis[J]. Oncogene, 2017, 36(27): 3797-3806. doi: 10.1038/onc.2017.6
[18] Fan J, Hitosugi T, Chung TW, et al. Tyrosine phosphorylation of lactate dehydrogenase A is important for NADH/NAD(+) redox homeostasis in cancer cells[J]. Mol Cell Biol, 2011, 31(24): 4938-4950. doi: 10.1128/MCB.06120-11
[19] Zhao D, Zou SW, Liu Y, et al. Lysine-5 acetylation negatively regulates lactate dehydrogenase A and is decreased in pancreatic cancer[J]. Cancer Cell, 2013, 23(4): 464-476. doi: 10.1016/j.ccr.2013.02.005
-




 下载:
下载: