The value of monitoring the expression level of soluble tumor suppressor factor 2 in predicting early severe acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation in children
-
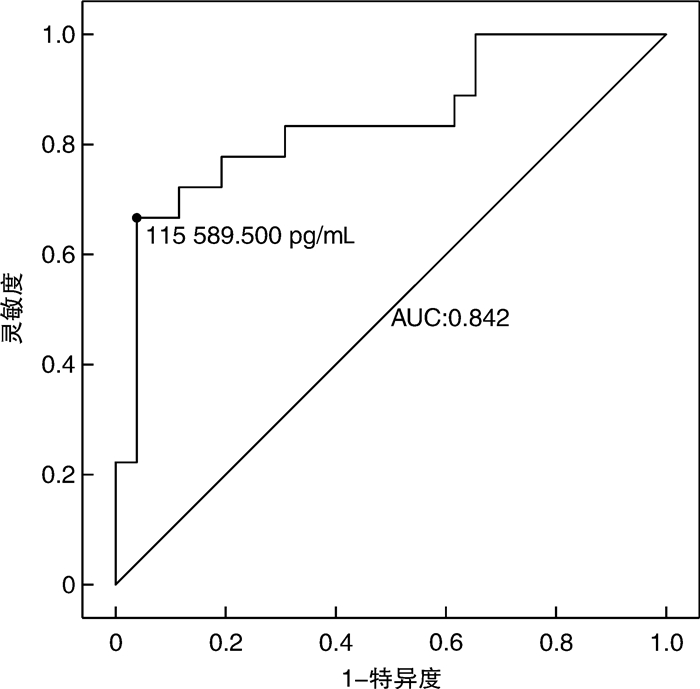
摘要: 目的 研究不同供者来源儿童异基因造血干细胞移植后监测外周血可溶性抑瘤因子2(soluble tumor suppressor factor 2,sST2)表达水平对早期重度急性移植物抗宿主病(acute graft-versus-host disease,aGVHD)发生的临床预测价值。方法 回顾性分析2020年1月至2022年5月在我院行allo-HSCT的44例患儿的临床资料,将移植后18例发生Ⅲ~Ⅳ度aGVHD的患儿作为观察组,其余26例患儿作为对照组,比较2组基线数据及患儿在移植前、移植后14天和21天sST2表达水平的差异。构建受试者工作特征曲线,根据sST2表达中位水平分为sST2高表达组和低表达组,比较2组患儿不同器官aGVHD的累积发生率。结果 观察组和对照组患儿移植前、移植后14天外周血sST2水平比较差异无统计学意义,观察组患儿移植后21天外周血sST2水平明显高于对照组,差异有统计学意义(P < 0.001)。移植后21天sST2诊断Ⅲ~Ⅳ度aGVHD的受试者工作特征曲线下面积为0.842(95%CI 0.715~0.968),对应的特异度为96%,灵敏度为67%。根据移植后21天外周血sST2表达水平的中位数(71 222.0 pg/mL),将患儿分为高sST2组(22例)和低sST2组(22例)。高sST2组Ⅲ~Ⅳ度aGVHD、2~4级肠道aGVHD、皮肤aGVHD发生率明显高于低sST2组(63.6% vs 18.2%,P=0.002;59.1% vs 13.6%,P=0.002;90.9% vs 63.6%,P=0.031);高sST2组上消化道或肝脏aGVHD发生率有高于低sST2组的趋势,但差异无统计学意义(40.9% vs 18.2%,P=0.099)。结论 血液病患儿不同供者来源异基因造血干细胞移植后21天外周血sST2表达水平升高有助于预测Ⅲ~Ⅳ度aGVHD的发生。外周血sST2有望成为异基因造血干细胞移植后重度aGVHD的生物标记物,仍需多中心、大样本进一步研究证实。Abstract: Objective To investigate the clinical value of monitoring the expression level of soluble tumor suppressor factor 2(sST2) in peripheral blood after allogeneic hematopoietic stem cell transplantation(allo-HSCT) in children from different donors in predicting the occurrence of early severe acute graft-versus-host disease(aGVHD).Methods The clinical data of 44 children who underwent allo-HSCT in our hospital from January 2020 to May 2022 were retrospectively analyzed. Eighteen children with grade Ⅲ-Ⅳ aGVHD after transplantation were selected as the observation group, and the remaining 26 children were selected as the control group. The baseline data and sST2 expression levels before allo-HSCT, 14 d and 21 d after allo-HSCT were compared between the two groups. The receiver operating characteristic curve was constructed. According to the median level of sST2 expression, the children were divided into a high sST2 expression group and a low sST2 expression group, and the cumulative incidence of aGVHD in different organs was compared between the two groups.Results There was no significant difference in the level of peripheral blood sST2 before transplantation and 14 days after allo-HSCT between the observation group and the control group. The level of peripheral blood sST2 in the observation group was significantly higher than that in the control group 21 days after transplantation(P < 0.001). The area under the receiver operating characteristic curve of sST2 for the diagnosis of grade Ⅲ-Ⅳ aGVHD at 21 days after allo-HSCT was 0.842(95%CI 0.715-0.968), and the corresponding sensitivity was 96%, the specificity was 67%. The children were divided into high sST2 group(22 cases) and low sST2 group(22 cases) according to the median level of sST2(71 222.0 pg/mL) in peripheral blood on day 21 after transplantation. The incidence of grade Ⅲ-ⅣaGVHD, grade 2-4 intestinal aGVHD, and skin aGVHD in the high sST2 group was significantly higher than that in the low sST2 group(63.6% vs 18.2%, P=0.002; 59.1% vs 13.6%, P=0.002; 90.9% vs 63.6%, P=0.031). The incidence of upper gastrointestinal or hepatic aGVHD also tended to be higher than that in the low sST2 group, while there was no significant difference(40.9% vs 18.2%, P=0.099).Conclusion The elevated expression of sST2 in peripheral blood of children with hematological diseases at 21 days after allo-HSCT from different donors is helpful to predict the occurrence of Ⅲ-Ⅳ aGVHD. Peripheral blood sST2 is expected to be a biomarker for severe aGVHD after allo-HSCT, which still needs to be further confirmed by multi-center and larger sample studies.
-

-
表 1 观察组和对照组患儿基本资料比较
临床特征 观察组
(18例)对照组
(26例)P 性别/例(%) 0.447 男 9(50.0) 16(61.5) 女 9(50.0) 10(38.5) 中位年龄(范围)/岁 5.5(1~14) 9(2~15) 0.060 中位中性粒细胞植入时间(范围)/d 15
(12~25)17.5
(11~28)0.479 原发疾病/例(%) 0.354 再生障碍性贫血 6(33.3) 6(23.1) 急性淋巴细胞白血病 2(11.1) 8(30.8) 急性髓系白血病 4(22.2) 9(34.6) 混合细胞性白血病 1(5.6) 0 骨髓增生异常综合征 2(11.1) 2(7.7) 幼年型粒单核细胞白血病 1(5.6) 0 丙酮酸激酶缺乏症 1(5.6) 0 先天性骨髓衰竭性疾病 1(5.6) 1(3.8) 供者来源/例(%) 0.528 同胞全相合 0 1(3.8) 单倍体 9(50.0) 8(30.8) 非血缘脐血 9(50.0) 17(65.4) HLA相合位点数/例(%) 0.369 5/10 7(38.9) 4(15.4) 6/10 2(11.1) 3(11.5) 7/10 3(16.7) 3(11.5) 8/10 2(11.1) 3(11.5) 9/10 3(16.7) 6(23.1) 10/10 1(5.6) 7(26.9) 预处理强度/例(%) 0.858 淸髓性预处理 12(66.7) 18(69.2) 减低强度预处理 6(33.3) 8(30.8) GVHD预防/例(%) 0.581 环孢素+吗替麦考酚酯+甲氨蝶呤 9(50.0) 8(30.8) 环孢素+吗替麦考酚酯 8(44.4) 14(53.8) 环孢素+甲氨蝶呤 0 1(3.8) 他克莫司+吗替麦考酚酯 1(5.6) 3(11.5) 细胞来源/例(%) 0.528 外周血 3(16.7) 4(15.4) 外周血+骨髓 6(33.3) 17(65.4) 脐血 9(50.0) 5(19.2) 表 2 观察组和对照组患儿移植前后sST2在外周血中表达水平比较
pg/mL sST2检测时间 观察组(18例) 对照组(26例) P 移植前(44例) 10 562.4(2 816.0~19 645.0) 9 760.4(637.0~32 878.0) 0.720 移植后14天(34例△) 48 018.0(10 394.0~611 375.0) 55 346.0(9 386.0~200 421.0) 0.876 移植后21天(44例) 125 421.0(29 167.9~494 025.0) 41 448.5(12 186.0~171 010.0) < 0.001 △移植后14天共34例患者检测外周血sST2水平,其中观察组15例,对照组19例。 表 3 高sST2组与低sST2组不同器官aGVHD的累积发生率比较
例(%) 不同器官aGVHD 高sST2组
(22例)低sST2组
(22例)P aGVHD 0.002 Ⅲ~Ⅳ度 14(63.6) 4(18.2) 0~Ⅱ度 8(36.4) 18(81.8) 肠道aGVHD 0.002 2~4级 13(59.1) 3(13.6) 0~1级 9(40.9) 19(86.4) 皮肤aGVHD 有 20(90.9) 14(63.6) 0.031 无 2(9.1) 8(36.4) 上消化道/肝脏aGVHD 有 9(40.9) 4(18.2) 0.099 无 13(59.1) 18(81.8) -
[1] Martini DJ, Chen YB, DeFilipp Z. Recent FDA Approvals in the Treatment of Graft-Versus-Host Disease[J]. Oncologist, 2022, 27(8): 685-693. doi: 10.1093/oncolo/oyac076
[2] 任瑞瑞, 马梁明, 王涛, 等. 单倍体与同胞相合异基因造血干细胞移植治疗恶性血液病疗效观察[J]. 临床血液学杂志, 2023, 36(1): 44-48. https://lcxy.whuhzzs.com/article/doi/10.13201/j.issn.1004-2806.2023.01.009
[3] 杜志丛, 赵艳丽, 曹星玉, 等. 单倍型供者造血干细胞移植治疗重型再生障碍性贫血的单中心研究[J]. 临床血液学杂志, 2023, 36(5): 366-372. https://lcxy.whuhzzs.com/article/doi/10.13201/j.issn.1004-2806.2023.05.013
[4] Bidgoli A, DePriest BP, Saatloo MV, et al. Current Definitions and Clinical Implications of Biomarkers in Graft-versus-Host Disease[J]. Transplant Cell Ther, 2022, 28(10): 657-666. doi: 10.1016/j.jtct.2022.07.008
[5] Major-Monfried H, Renteria AS, Pawarode A, et al. MAGIC biomarkers predict long-term outcomes for steroid-resistant acute GVHD[J]. Blood, 2018, 131(25): 2846-2855. doi: 10.1182/blood-2018-01-822957
[6] Paczesny S. Post-haematopoietic cell transplantation outcomes: why ST2 became a 'golden nugget' biomarker[J]. Br J Haematol, 2021, 192(6): 951-967. doi: 10.1111/bjh.16497
[7] Rowan CM, Pike F, Cooke KR, et al. Assessment of ST2 for risk of death following graft-versus-host disease in pediatric and adult age groups[J]. Blood, 2020, 135(17): 1428-1437. doi: 10.1182/blood.2019002334
[8] Schoemans HM, Lee SJ, Ferrara JL, et al. EBMT-NIH-CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment[J]. Bone Marrow Transplant, 2018, 53(11): 1401-1415. doi: 10.1038/s41409-018-0204-7
[9] 中华医学会血液学分会干细胞应用学组. 中国异基因造血干细胞移植治疗血液系统疾病专家共识(Ⅲ)——急性移植物抗宿主病(2020年版)[J]. 中华血液学杂志, 2020, 41(7): 529-536.
[10] Hotta M, Satake A, Yoshimura H, et al. Elevation of Early Plasma Biomarkers in Patients with Clinical Risk Factors Predicts Increased Nonrelapse Mortality after Allogeneic Hematopoietic Stem Cell Transplantation[J]. Transplant Cell Ther, 2021, 27(8): 660.e1-660.e8. doi: 10.1016/j.jtct.2021.04.025
[11] Zeiser R, Blazar BR. Acute Graft-versus-Host Disease-Biologic Process, Prevention, and Therapy[J]. N Engl J Med, 2017, 377(22): 2167-2179. doi: 10.1056/NEJMra1609337
[12] Homsak E, Gruson D. Soluble ST2: A complex and diverse role in several diseases[J]. Clin Chim Acta, 2020, 507: 75-87. doi: 10.1016/j.cca.2020.04.011
[13] Ramadan AM, Daguindau E, Rech JC, et al. From proteomics to discovery of first-in-class ST2 inhibitors active in vivo[J]. JCI insight, 2018, 3(14): e99208. doi: 10.1172/jci.insight.99208
[14] Balakrishnan B, Kulkarni UP, Pai AA, et al. Biomarkers for early complications post hematopoietic cell transplantation: Insights and challenges[J]. Front Immunol, 2023, 14: 1100306.
[15] Matsumura A, Miyazaki T, Tachibana T, et al. Predictive Values of Early Suppression of Tumorigenicity 2 for Acute GVHD and Transplant-related Complications after Allogeneic Stem Cell Transplantation: Prospective Observational Study[J]. Turk J Haematol, 2020, 37(1): 20-29.
[16] 张爱萍, 熊昊, 王卓, 等. 儿童异基因造血干细胞移植后血浆sST2/Reg3α蛋白水平与急性移植物抗宿主病的相关性[J]. 中国实验血液学杂志, 2021, 29(4): 1334-1339. https://www.cnki.com.cn/Article/CJFDTOTAL-XYSY202104053.htm
[17] Zhang J, Ramadan AM, Griesenauer B, et al. ST2 blockade reduces sST2-producing T cells while maintaining protective mST2-expressing T cells during graft-versus-host disease[J]. Sci Transl Med, 2015, 7(308): 308ra160.
[18] 温建芸, 苗丽丽, 管迪, 等. 血浆生物标记物水平与儿童急性移植物抗宿主病的相关性[J]. 中国组织工程研究, 2022, 26(31): 5032-5039. https://www.cnki.com.cn/Article/CJFDTOTAL-XDKF202231021.htm
[19] 王叨, 王玮琳, 丁艳杰, 等. 人再生胰岛衍生蛋白、可溶性抑瘤因子2、肿瘤坏死因子受体1在儿童异基因造血干细胞移植术后肠道急性移植物抗宿主病诊疗中的价值[J]. 中华实用儿科临床杂志, 2022, 37(13): 992-996.
-




 下载:
下载: