Expression and clinical significance of TIGIT, CD226, CD96, and CD155 in platelet glycoprotein autoantibody-positive primary immune thrombocytopenia
-
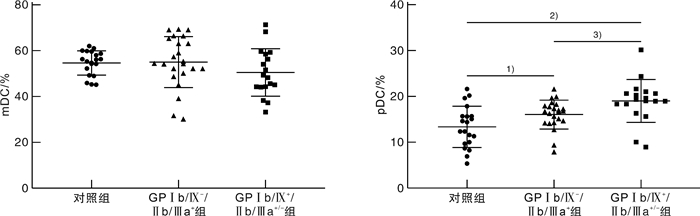
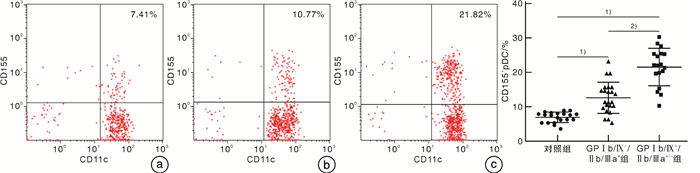
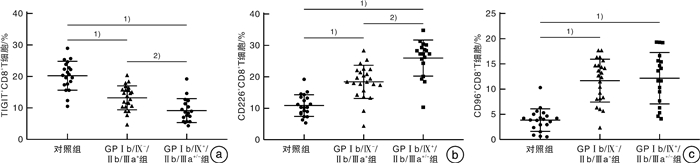
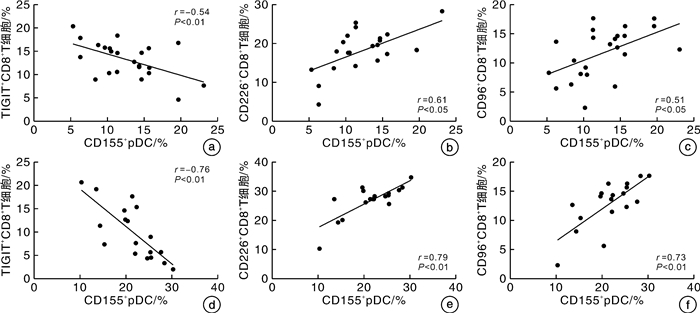
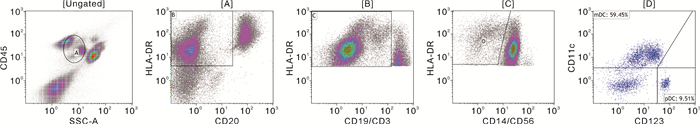
摘要: 目的 探讨TIGIT、CD226、CD96和CD155在血小板糖蛋白自身抗体(platelet glycoprotein autoantibody,GPs)阳性的原发性免疫性血小板减少症(immune thrombocytopenia,ITP)患者外周血CD8+T细胞和树突状细胞(dendritic cell,DC)膜表面的表达,并分析其临床意义。方法 选取41例GPs阳性的ITP患者和20例健康对照者,采用流式细胞术检测CD8+T细胞表面TIGIT、CD226和CD96表达比例,并检测ITP患者外周血DC细胞的亚群变化及浆细胞样DC(plasma DC,pDC)上CD155的表达比例。结果 GPs阳性ITP组患者的pDC和CD155+pDC比例均明显高于对照组,且GPⅠb/Ⅸ+/Ⅱb/Ⅲa+/-组均明显高于GPⅠb/Ⅸ-/Ⅱb/Ⅲa+组(P < 0.05,F=40.83;P < 0.01,F=59.50)。GPs阳性ITP组患者的TIGIT+CD8+T细胞比例明显低于对照组,且GPⅠb/Ⅸ+/Ⅱb/Ⅲa+/-组明显低于GPⅠb/Ⅸ-/Ⅱb/Ⅲa+组(P < 0.05,F=36.41),但CD226+CD8+T细胞与CD96+CD8+T细胞比例均明显高于对照组,且GPⅠb/Ⅸ+/Ⅱb/Ⅲa+/-组均明显高于GPⅠb/Ⅸ-/Ⅱb/Ⅲa+组(P < 0.05,F=45.01;P < 0.01,F=41.66)。GPⅠb/Ⅸ-/Ⅱb/Ⅲa+组与GPⅠb/Ⅸ+/Ⅱb/Ⅲa+/-组的CD155+pDC与TIGIT+CD8+T细胞比例呈负相关(P < 0.01,r=-0.54;P < 0.01,r=-0.76),与CD226+CD8+T细胞和CD96+CD8+T细胞比例呈正相关(P < 0.05,r=0.61;P < 0.05,r=0.51;P < 0.01,r=0.79;P < 0.01,r=0.73)。结论 TIGIT、CD226、CD96与CD155表达异常,导致GPs阳性的ITP的免疫失耐受,可能参与了疾病发生。Abstract: Objective To investigate the expression of TIGIT, CD226, CD96 and CD155 on CD8+T cells and dendritic cells(DC) in the peripheral blood of patients with platelet glycoprotein autoantibody(GPs) -positive primary immune thrombocytopenia(ITP) and to analyze their clinical significance.Methods Forty-one GP-positive ITP patients and 20 healthy individuals were selected. The flow cytometry was used to detect the percentage of TIGIT, CD226, and CD96 expression on CD8+T cells and the changes in DC cell subsets and the percentage of CD155 expression on plasma DC(pDC) were analyzed.Results The percentage of pDC and CD155+pDC in the GPs-positive ITP group were significantly higher than those in the healthy control group, and they were significantly higher in the GPⅠb/Ⅸ+/Ⅱb/Ⅲa+/-group than those in the GPⅠb/Ⅸ-/Ⅱb/Ⅲa+group(P < 0.05, F=40.83; P < 0.01, F=59.50). On the other hand, the percentage of TIGIT+CD8+T cells in the GPs-positive ITP group was significantly lower than that in the healthy control group, and it was significantly lower in the GPⅠb/Ⅸ+/Ⅱb/Ⅲa+/-group than that in the GPⅠb/Ⅸ-/Ⅱb/Ⅲa+group(P < 0.05, F=36.41). However, the percentages of CD226+CD8+T cells and CD96+CD8+T cells were significantly higher than those in the control group, and they were significantly higher in the GPⅠb/Ⅸ+/Ⅱb/Ⅲa+/-group than those in the GPⅠb/Ⅸ-/Ⅱb/Ⅲa+group(P < 0.05, F=45.01; P < 0.01, F=41.66). The percentages of CD155+pDC in the GPⅠb/Ⅸ-/Ⅱb/Ⅲa+group and GPⅠb/Ⅸ+/Ⅱb/Ⅲa+/-group were negatively correlated with TIGIT+CD8+T cells(P < 0.01, r=-0.54; P < 0.01, r=-0.76), while it positively correlated with the percentages of CD226+CD8+T cells and CD96+CD8+T cells(P < 0.05, r=0.61; P < 0.05, r=0.51; P < 0.01, r=0.79; P < 0.01, r=0.73).Conclusion The abnormal expression of TIGIT, CD226, CD96, and CD155 contributes to immune intolerance in GPs-positive ITP, which may be involved in the pathogenesis of the disease.
-
Key words:
- immune thrombocytopenia /
- inhibitory molecule /
- CD226 /
- CD96
-

-
表 1 GPⅠb/Ⅸ+/Ⅱb/Ⅲa-组与GPⅠb/Ⅸ+/Ⅱb/Ⅲa+组DC亚群、CD155+pDC和CD226+/TIGIT+/CD96+CD8+T细胞比例的比较
组别 例数 pDC/% mDC/% CD155+pDC/% CD226+
CD8+T/%TIGIT+
CD8+T/%CD96+
CD8+T/%GPⅠb/Ⅸ+/Ⅱb/Ⅲa-组 13 18.82
(16.32,21.02)46.93
(44.32,58.51)21.75
(19.86,25.32)27.31
(26.21,29.32)7.49
(5.69,15.36)13.21
(12.65,15.65)GPⅠb/Ⅸ+/Ⅱb/Ⅲa+组 5 19.03
(18.96,19.03)51.63
(44.18,57.82)22.01
(17.48,25.41)27.32
(22.23,30.28)8.21
(4.95,13.65)14.23
(10.94,15.48)Z -0.093 -0.246 -0.049 -0.542 -0.197 -0.187 P 0.926 0.805 0.961 0.588 0.844 0.766 -
[1] Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia[J]. Blood Adv, 2019, 3(23): 3829-3866. doi: 10.1182/bloodadvances.2019000966
[2] Singh A, Uzun G, Bakchoul T. Primary Immune Thrombocytopenia: Novel Insights into Pathophysiology and Disease Management[J]. J Clin Med, 2021, 10(4): 789. doi: 10.3390/jcm10040789
[3] 桑海强, 马慧慧, 冯蕊涵. 替罗非班诱导的血小板减少症对患者预后影响的回顾性对照研究[J]. 临床心血管病杂志, 2022, 38(2): 113-118. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXB202202007.htm
[4] Vrbensky JR, Nazy I, Clare R, et al. T cell-mediated autoimmunity in immune thrombocytopenia[J]. Eur J Haematol, 2022, 108(1): 18-27. doi: 10.1111/ejh.13705
[5] Wen R, Wang Y, Hong Y, et al. Cellular immune dysregulation in the pathogenesis of immune thrombocytopenia[J]. Blood Coagul Fibrinolysis, 2020, 31(2): 113-120. doi: 10.1097/MBC.0000000000000891
[6] Audia S, Mahevas M, Nivet M, et al. Immune Thrombocytopenia: Recent Advances in Pathogenesis and Treatments[J]. Hemasphere, 2021, 5(6): e574. doi: 10.1097/HS9.0000000000000574
[7] Xu SQ, Wang CY, Zhu XJ, et al. Decreased indoleamine 2, 3-dioxygenase expression in dendritic cells and role of indoleamine 2, 3-dioxygenase-expressing dendritic cells in immune thrombocytopenia[J]. Ann Hematol, 2012, 91(10): 1623-1631. doi: 10.1007/s00277-012-1451-0
[8] Harjunpaa H, Guillerey C. TIGIT as an emerging immune checkpoint[J]. Clin Exp Immunol, 2020, 200(2): 108-119. doi: 10.1111/cei.13407
[9] 侯明, 刘新光. 立足中国实际的原发免疫性血小板减少症诊治——2020版成人原发免疫性血小板减少症诊断与治疗中国指南解读[J]. 临床血液学杂志, 2021, 34(1): 1-4. https://lcxy.whuhzzs.com/article/doi/10.13201/j.issn.1004-2806.2021.01.001
[10] Zhao Z, Yang L, Yang G, et al. Contributions of T lymphocyte abnormalities to therapeutic outcomes in newly diagnosed patients with immune thrombocytopenia[J]. PLoS One, 2015, 10(5): e126601.
[11] Audia S, Mahevas M, Samson M, et al. Pathogenesis of immune thrombocytopenia[J]. Autoimmun Rev, 2017, 16(6): 620-632. doi: 10.1016/j.autrev.2017.04.012
[12] Yu TS, Wang HY, Zhao YJ, et al. Abnormalities of bone marrow B cells and plasma cells in primary immune thrombocytopenia[J]. Blood Adv, 2021, 5(20): 4087-4101. doi: 10.1182/bloodadvances.2020003860
[13] Bakchoul T, Walek K, Krautwurst A, et al. Glycosylation of autoantibodies: insights into the mechanisms of immune thrombocytopenia[J]. Thromb Haemost, 2013, 110(6): 1259-1266.
[14] Li J, van der Wal DE, Zhu G, et al. Desialylation is a mechanism of Fc-independent platelet clearance and a therapeutic target in immune thrombocytopenia[J]. Nat Commun, 2015, 6: 7737. doi: 10.1038/ncomms8737
[15] Tao L, Zeng Q, Li J, et al. Platelet desialylation correlates with efficacy of first-line therapies for immune thrombocytopenia[J]. J Hematol Oncol, 2017, 10(1): 46. doi: 10.1186/s13045-017-0413-3
[16] Qiu J, Liu X, Li X, et al. CD8(+)T cells induce platelet clearance in the liver via platelet desialylation in immune thrombocytopenia[J]. Sci Rep, 2016, 6: 27445. doi: 10.1038/srep27445
[17] Quach ME, Dragovich MA, Chen W, et al. Fc-independent immune thrombocytopenia via mechanomolecular signaling in platelets[J]. Blood, 2018, 131(7): 787-796. doi: 10.1182/blood-2017-05-784975
[18] Olsson B, Jernas M, Wadenvik H. Increased plasma levels of granzymes in adult patients with chronic immune thrombocytopenia[J]. Thromb Haemost, 2012, 107(6): 1182-1184. doi: 10.1160/TH12-01-0012
[19] Marini I, Zlamal J, Faul C, et al. Autoantibody-mediated desialylation impairs human thrombopoiesis and platelet lifespan[J]. Haematologica, 2021, 106(1): 196-207.
[20] 周婕, 徐敏, 黄琳琳, 等. 血小板特异性自身抗体在免疫性血小板减少症患者的临床特征和预后评估中的作用[J]. 临床血液学杂志, 2022, 35(7): 469-473. https://lcxy.whuhzzs.com/article/doi/10.13201/j.issn.1004-2806.2022.07.004
[21] Mason KD, Carpinelli MR, Fletcher JI, et al. Programmed anuclear cell death delimits platelet life span[J]. Cell, 2007, 128(6): 1173-1186. doi: 10.1016/j.cell.2007.01.037
[22] Zhang X, Wang Y, Zhang D, et al. CD70-silenced dendritic cells induce immune tolerance in immune thrombocytopenia patients[J]. Br J Haematol, 2020, 191(3): 466-475. doi: 10.1111/bjh.16689
[23] 黎晓鹃, 李晓双. ITP患者外周血DC亚群和CD80、CD86表达变化及其与地塞米松治疗效果的相关性分析[J]. 中国实验血液学杂志, 2018, 26(6): 1752-1756. https://www.cnki.com.cn/Article/CJFDTOTAL-XYSY201806032.htm
[24] Semple JW, Rebetz J, Maouia A, et al. An update on the pathophysiology of immune thrombocytopenia[J]. Curr Opin Hematol, 2020, 27(6): 423-429. doi: 10.1097/MOH.0000000000000612
[25] Liu J, Zhang X, Cao X. Dendritic cells in systemic lupus erythematosus: From pathogenesis to therapeutic applications[J]. J Autoimmun, 2022, 132: 102856. doi: 10.1016/j.jaut.2022.102856
[26] Malik A, Sayed AA, Han P, et al. The role of CD8+ T-cell clones in immune thrombocytopenia[J]. Blood, 2023, 141(20): 2417-2429.
[27] Zhou H, Qiu JH, Wang T, et al. Interleukin 27 inhibits cytotoxic T-lymphocyte-mediated platelet destruction in primary immune thrombocytopenia[J]. Blood, 2014, 124(22): 3316-3319. doi: 10.1182/blood-2014-06-580084
[28] Sanchez-Correa B, Gayoso I, Bergua JM, et al. Decreased expression of DNAM-1 on NK cells from acute myeloid leukemia patients[J]. Immunol Cell Biol, 2012, 90(1): 109-115. doi: 10.1038/icb.2011.15
[29] Jin HS, Park Y. Hitting the complexity of the TIGIT-CD96-CD112R-CD226 axis for next-generation cancer immunotherapy[J]. BMB Rep, 2021, 54(1): 2-11. doi: 10.5483/BMBRep.2021.54.1.229
[30] Chiang EY, de Almeida PE, de Almeida ND, et al. CD96 functions as a co-stimulatory receptor to enhance CD8(+)T cell activation and effector responses[J]. Eur J Immunol, 2020, 50(6): 891-902. doi: 10.1002/eji.201948405
[31] Song G, Bae SC, Choi S, et al. Association between the CD226 rs763361 polymorphism and susceptibility to autoimmune diseases: a meta-analysis[J]. Lupus, 2012, 21(14): 1522-1530. doi: 10.1177/0961203312458840
[32] Braun M, Aguilera AR, Sundarrajan A, et al. CD155 on Tumor Cells Drives Resistance to Immunotherapy by Inducing the Degradation of the Activating Receptor CD226 in CD8(+)T Cells[J]. Immunity, 2020, 53(4): 805-823. doi: 10.1016/j.immuni.2020.09.010
[33] Liu L, You X, Han S, et al. CD155/TIGIT, a novel immune checkpoint in human cancers(Review)[J]. Oncol Rep, 2021, 45(3): 835-845. doi: 10.3892/or.2021.7943
[34] Wu L, Mao L, Liu JF, et al. Blockade of TIGIT/CD155 Signaling Reverses T-cell Exhaustion and Enhances Antitumor Capability in Head and Neck Squamous Cell Carcinoma[J]. Cancer Immunol Res, 2019, 7(10): 1700-1713. doi: 10.1158/2326-6066.CIR-18-0725
[35] Sun H, Huang Q, Huang M, et al. Human CD96 Correlates to Natural Killer Cell Exhaustion and Predicts the Prognosis of Human Hepatocellular Carcinoma[J]. Hepatology, 2019, 70(1): 168-183. doi: 10.1002/hep.30347
[36] Lepletier A, Lutzky VP, Mittal D, et al. The immune checkpoint CD96 defines a distinct lymphocyte phenotype and is highly expressed on tumor-infiltrating T cells[J]. Immunol Cell Biol, 2019, 97(2): 152-164.
-




 下载:
下载: