Genetic heterogeneity in core-binding factor acute myeloid leukemia and its clinical implication
-
摘要: 目的 探讨成人核心结合因子急性髓系白血病(core-binding factor acute myeloid leukemia,CBF-AML)的遗传异质性及预后因素。方法 回顾性分析271例成人新诊断CBF-AML患者的临床资料,包括188例t(8;21) AML患者和83例inv(16)/t(16;16) AML患者。比较2组患者间分子遗传学差异,采用log-rank检验和Cox回归模型分析影响患者生存和复发的因素。结果 t(8;21) AML患者性染色体缺失(33.6% vs 1.5%,P<0.001)、CD19(58.9% vs 6.8%,P<0.001)和CD56表达(63.8% vs 1.7%,P<0.001)明显高于inv(16)/t(16;16) AML患者。+22在inv(16)/t(16;16) AML患者中明显高于t(8;21) AML患者(13.6% vs 0.7%,P<0.001)。 KIT 突变(51.8% vs 28.3%,P=0.010)、 EZH2突变 (18.8% vs 4.3%,P=0.022)在t(8;21) AML患者中的发生率明显高于inv(16)/t(16;16) AML患者。 FLT3 突变(34.8% vs 12.9%,P=0.003)和 WT1突变 (15.2% vs 4.7%,P=0.044)在inv(16)/t(16;16) AML患者中的发生率明显高于t(8;21) AML患者。对于t(8;21) AML患者, KIT D816 突变是影响总生存的独立危险因素(P=0.050),而异基因造血干细胞移植是影响总生存的独立保护因素(P=0.029)。初诊时骨髓高原始细胞数(P=0.043)、CD19不表达(P=0.008)是影响无事件生存的独立危险因素。 KIT D816 突变(P=0.014)、CD19不表达(P=0.036)是影响累计复发率的独立危险因素。对于inv(16)/t(16;16) AML患者,髓外浸润是影响无事件生存的独立危险因素(P=0.023),异基因造血干细胞移植是影响累计复发率(P=0.037)和无事件生存(P=0.015)的独立保护因素。结论 成人t(8;21)和inv(16)/t(16;16) AML患者具有显著的遗传学异质性。
-
关键词:
- 成人核心结合因子相关急性髓系白血病 /
- t(8;21) /
- inv(16)/t(16;16) /
- 异质性
Abstract: Objective To explore the genetic heterogeneity and prognostic factors in adult core-binding factor acute myeloid leukemia(CBF-AML).Methods The clinical data of 271 newly diagnosed adult CBF-AML were retrospectively analyzed, including 188 patients with t(8; 21) AML and 83 patients with inv(16)/t(16; 16) AML. Chi-square test was used to compare the difference of molecular genetic between t(8; 21) AML and inv(16)/t(16; 16) AML. Log-rank test and Cox regression model were used to analyze the impact of clinical factors and gene mutations on survival and relapse in CBF-AML.Results Sex chromosome deletion, CD19 expression, and CD56 expression were more common in t(8; 21) AML(33.6% vs 1.5%, P<0.001; 58.9% vs 6.8%, P<0.001; 63.8% vs 1.7%, P<0.001), while trisomy 22 was more common in inv(16)/t(16; 16) AML(13.6% vs 0.7%, P<0.001). The incidences of KIT and EZH2 mutations in t(8; 21) AML were significantly higher than those in inv(16)/t(16; 16) AML(51.8% vs 28.3%, P=0.010; 18.8% vs 4.3%, P=0.022). The incidences of FLT3 and WT1 mutations were significantly higher in inv(16)/t(16; 16) AML than those in t(8; 21) AML(34.8% vs 12.9%, P=0.003; 15.2% vs 4.7%, P=0.044). For t(8; 21) AML patients, KIT D816 was an independent risk factor for overall survival(P=0.050) and allogeneic hematopoietic stem cell transplantation was an independent protective factor for overall survival(P=0.029). Higher bone marrow blasts and CD19 negative were independent risk factors for event-free survival(P=0.043; P=0.008). KIT D816 and CD19 negative were independent risk factors for cumulative incidence of relapse(P=0.014; P=0.036). For inv(16)/t(16; 6) AML patients, extramedullary involvement was the independent risk factor for event-free survival(P=0.023) and allogeneic hematopoietic stem cell transplantation was the independent protective factor for cumulative incidence of relapse(P=0.037) and event-free survival(P=0.015).Conclusion t(8; 21) and inv(16)/t(16; 16) AML are heterogeneous in clinical characteristics, cytogenetics, gene mutation profile, and prognostic factors.-
Key words:
- core-binding factor acute myeloid leukemia /
- t(8; 21) /
- inv(16)/t(16; 16) /
- heterogeneity
-

-
表 1 CBF-AML患者的临床特征
临床特征 所有患者
(271例)t(8;21) AML
(188例)inv(16)/t(16;16) AML
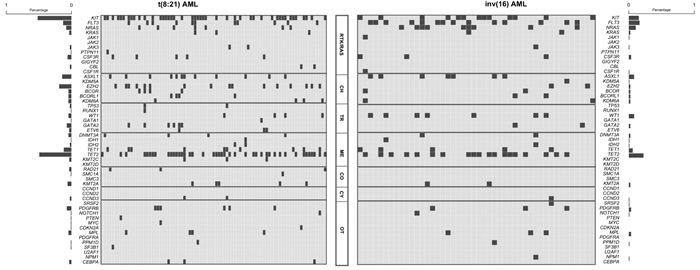
(83例)P 年龄/岁 34(26,45) 34(24,45) 34(27,46) 0.345 男∶女/例 165∶106 108∶80 57∶26 0.081 AML类型/例(%) 0.674 原发AML 263(97.0) 183(97.3) 80(96.4) 继发AML 8(3.0) 5(2.7) 3(3.6) 白细胞计数/(×109/L) 15.42(7.13,37.91) 10.80(5.00,23.27) 50.00(22.38,103.60) <0.001 骨髓原始细胞/% 46.91(30.50,64.00) 43.00(29.38,59.13) 55.44(38.00,70.00) 0.003 CD19表达/例(%) 87(43.5) 83(58.9) 4(6.8) <0.001 CD33表达/例(%) 168(84.0) 114(80.9) 54(91.5) 0.060 CD56表达/例(%) 91(45.5) 90(63.8) 1(1.7) <0.001 髓外浸润/例(%) 24(8.9) 19(10.1) 5(6.0) 0.276 附加染色体异常/例(%) 89(42.6) 68(47.6) 21(31.8) 0.032 X/Y性染色体缺失 49(23.4) 48(33.6) 1(1.5) <0.001 +8染色体三体 5(2.4) 2(1.4) 3(4.5) 0.186 +22染色体三体 10(4.8) 1(0.7) 9(13.6) <0.001 9q/-9染色体缺失 7(3.3) 7(4.9) 0 0.020 7q/-7染色体缺失 10(4.8) 8(5.6) 2(3.0) 0.401 复杂核型 13(6.2) 8(5.6) 5(7.6) 0.587 分子遗传学异常/例(%) TET2突变 61(46.6) 42(49.4) 19(41.3) 0.375 KIT突变 57(43.5) 44(51.8) 13(28.3) 0.010 KIT exon 8突变 5(3.8) 3(3.5) 2(4.3) 0.817 KITexon 17突变 50(38.2) 40(47.1) 10(21.7) 0.004 FLT3突变 27(20.6) 11(12.9) 16(34.8) 0.003 FLT3-ITD突变 12(9.2) 6(7.1) 6(13.0) 0.267 FLT3-TKD突变 12(9.2) 5(5.9) 7(15.2) 0.085 ASXL1突变 19(14.6) 12(14.1) 7(15.6) 0.825 EZH2突变 18(13.7) 16(18.8) 2(4.3) 0.022 NRAS突变 17(13.0) 7(8.2) 10(21.7) 0.028 TET1突变 15(11.5) 10(11.8) 5(10.9) 0.878 WT1突变 11(8.4) 4(4.7) 7(15.2) 0.044 缓解后治疗方式/例(%) 化疗 88(34.0) 61(34.1) 27(33.8) 0.959 自体造血干细胞移植 43(16.6) 23(12.8) 20(25.0) 0.015 异基因造血干细胞移植 128(49.4) 95(53.1) 33(41.2) 0.079 表 2 t(8;21) AML患者预后多因素分析
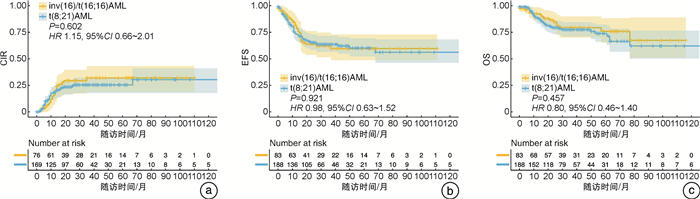
相关因素 OS CIR EFS HR(95%CI) P HR(95%CI) P HR(95%CI) P 白细胞计数≥15.42×109/L - - - - 2.85(0.05~5.67) 0.290 骨髓原始细胞≥46.91% 2.30(0.89~5.98) 0.085 - - 2.57(1.05~3.56) 0.043 KIT D816突变 2.56(1.00~6.61) 0.050 5.75(1.43~23.15) 0.014 1.01(0.08~13.19) 0.994 CD19表达 - - 0.33(0.11~0.93) 0.036 0.15(0.03~0.62) 0.008 2个疗程后达CR 2.64(0.83~8.33) 0.098 1.69(0.30~9.54) 0.552 0.01(0~2.78) 0.112 3个疗程后融合基因水平<0.1% - - 0.33(0.08~1.40) 0.132 0.48(0~24.316) 0.714 异基因造血干细胞移植 0.35(0.14~0.90) 0.029 0.64(0.12~3.42) 0.601 0.15(0.01~1.78) 0.131 表 3 inv(16)/t(16;16) AML患者预后多因素分析
相关因素 OS CIR EFS HR(95%CI) P HR(95%CI) P HR(95%CI) P 白细胞计数≥15.42×109/L - - 3.68(0.83~16.45) 0.088 - - 髓外浸润 0.88(0.35~2.46) 0.989 2.62(0.73~9.43) 0.141 3.52(1.19~10.44) 0.023 FLT3-ITD突变 2.37(0.48~11.76) 0.290 - - - - 3个疗程后融合基因水平<0.1% 0.48(0.12~1.96) 0.310 - - - - 异基因造血干细胞移植 0.38(0.09~1.55) 0.178 0.37(0.15~0.94) 0.037 0.38(0.18~0.83) 0.015 -
[1] Al-Harbi S, Aljurf M, Mohty M, et al. An update on the molecular pathogenesis and potential therapeutic targeting of AML with t(8;21)(q22;q22.1);RUNX1-RUNX1T1[J]. Blood Adv, 2020, 4(1): 229-238. doi: 10.1182/bloodadvances.2019000168
[2] Jahn N, Terzer T, Sträng E, et al. Genomic heterogeneity in core-binding factor acute myeloid leukemia and its clinical implication[J]. Blood Adv, 2020, 4(24): 6342-6352. doi: 10.1182/bloodadvances.2020002673
[3] Cher CY, Leung GM, Au CH, et al. Next-generation sequencing with a myeloid gene panel in core-binding factor AML showed KIT activation loop and TET2 mutations predictive of outcome[J]. Blood Cancer J, 2016, 6(7): e442. doi: 10.1038/bcj.2016.51
[4] Park S, Choi H, Kim HJ, et al. Genome-wide genotype-based risk model for survival in core binding factor acute myeloid leukemia patients[J]. Ann Hematol, 2018, 97(6): 955-965. doi: 10.1007/s00277-018-3260-6
[5] Mosna F, Papayannidis C, Martinelli G, et al. Complex karyotype, older age, and reduced first-line dose intensity determine poor survival in core binding factor acute myeloid leukemia patients with long-term follow-up[J]. Am J Hematol, 2015, 90(6): 515-523. doi: 10.1002/ajh.24000
[6] Yu S, Lin T, Nie D, et al. Dynamic assessment of measurable residual disease in favorable-risk acute myeloid leukemia in first remission, treatment, and outcomes[J]. Blood Cancer J, 2021, 11(12): 195. doi: 10.1038/s41408-021-00591-4
[7] Xu L, Chen H, Chen J, et al. The consensus on indications, conditioning regimen, and donor selection of allogeneic hematopoietic cell transplantation for hematological diseases in China-recommendations from the Chinese Society of Hematology[J]. J Hematol Oncol, 2018, 11(1): 33. doi: 10.1186/s13045-018-0564-x
[8] Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN[J]. Blood, 2022, 140(12): 1345-1377. doi: 10.1182/blood.2022016867
[9] Shang L, Chen X, Liu Y, et al. The immunophenotypic characteristics and flow cytometric scoring system of acute myeloid leukemia with t(8;21)(q22;q22);RUNX1-RUNX1T1[J]. Int J Lab Hematol, 2019, 41(1): 23-31. doi: 10.1111/ijlh.12916
[10] Walter K, Cockerill PN, Barlow R, et al. Aberrant expression of CD19 in AML with t(8;21) involves a poised chromatin structure and PAX5[J]. Oncogene, 2010, 29(20): 2927-2937. doi: 10.1038/onc.2010.56
[11] Iriyama N, Hatta Y, Takeuchi J, et al. CD56 expression is an independent prognostic factor for relapse in acute myeloid leukemia with t(8;21)[J]. Leuk Res, 2013, 37(9): 1021-1026. doi: 10.1016/j.leukres.2013.05.002
[12] Wang B, Yang B, Ling Y, et al. Role of CD19 and specific KIT-D816 on risk stratification refinement in t(8;21) acute myeloid leukemia induced with different cytarabine intensities[J]. Cancer Med, 2021, 10(3): 1091-1102. doi: 10.1002/cam4.3705
[13] Sakamoto K, Shiba N, Deguchi T, et al. Negative CD19 expression is associated with inferior relapse-free survival in children with RUNX1-RUNX1T1-positive acute myeloid leukaemia: results from the Japanese Paediatric Leukaemia/Lymphoma Study Group AML-05 study[J]. Br J Haematol, 2019, 187(3): 372-376. doi: 10.1111/bjh.16080
[14] Hsiao HH, Liu YC, Wang HC, et al. Additional chromosomal abnormalities in core-binding factor acute myeloid leukemia[J]. Genet Mol Res, 2015, 14(4): 17028-17033. doi: 10.4238/2015.December.16.3
[15] Schlenk RF, Benner A, Krauter J, et al. Individual patient data-based meta-analysis of patients aged 16 to 60 years with core binding factor acute myeloid leukemia: a survey of the German Acute Myeloid Leukemia Intergroup[J]. J Clin Oncol, 2004, 22(18): 3741-3750. doi: 10.1200/JCO.2004.03.012
[16] Krauth MT, Eder C, Alpermann T, et al. High number of additional genetic lesions in acute myeloid leukemia with t(8;21)/RUNX1-RUNX1T1: frequency and impact on clinical outcome[J]. Leukemia, 2014, 28(7): 1449-1458. doi: 10.1038/leu.2014.4
[17] Wang YY, Zhao LJ, Wu CF, et al. C-KIT mutation cooperates with full-length AML1-ETO to induce acute myeloid leukemia in mice[J]. Proc Natl Acad Sci U S A, 2011, 108(6): 2450-2455. doi: 10.1073/pnas.1019625108
[18] Duployez N, Marceau-Renaut A, Boissel N, et al. Comprehensive mutational profiling of core binding factor acute myeloid leukemia[J]. Blood, 2016, 127(20): 2451-2459. doi: 10.1182/blood-2015-12-688705
[19] Opatz S, Bamopoulos SA, Metzeler KH, et al. The clinical mutatome of core binding factor leukemia[J]. Leukemia, 2020, 34(6): 1553-1562. doi: 10.1038/s41375-019-0697-0
[20] Faber ZJ, Chen X, Gedman AL, et al. The genomic landscape of core-binding factor acute myeloid leukemias[J]. Nat Genet, 2016, 48(12): 1551-1556. doi: 10.1038/ng.3709
[21] 吴天梅, 薛胜利, 李正, 等. KIT及其他克隆性基因突变对核心结合因子相关急性髓系白血病的预后价值[J]. 中华血液学杂志, 2021, 42(8): 646-653.
[22] Ayatollahi H, Shajiei A, Sadeghian MH, et al. Prognostic Importance of C-KIT Mutations in Core Binding Factor Acute Myeloid Leukemia: A Systematic Review[J]. Hematol Oncol Stem Cell Ther, 2017, 10(1): 1-7. doi: 10.1016/j.hemonc.2016.08.005
[23] Badr P, Elsayed GM, Eldin DN, et al. Detection of KIT mutations in core binding factor acute myeloid leukemia[J]. Leuk Res Rep, 2018, 10: 20-25.
[24] 中华医学会血液学分会白血病淋巴瘤学组. 中国成人急性髓系白血病(非急性早幼粒细胞白血病)诊疗指南(2021年版)[J]. 中华血液学杂志, 2021, 42(8): 617-623.
[25] Ishikawa Y, Kawashima N, Atsuta Y, et al. Prospective evaluation of prognostic impact of KIT mutations on acute myeloid leukemia with RUNX1-RUNX1T1 and CBFB-MYH11[J]. Blood Adv, 2020, 4(1): 66-75. doi: 10.1182/bloodadvances.2019000709
[26] Yui S, Kurosawa S, Yamaguchi H, et al. D816 mutation of the KIT gene in core binding factor acute myeloid leukemia is associated with poorer prognosis than other KIT gene mutations[J]. Ann Hematol, 2017, 96(10): 1641-1652. doi: 10.1007/s00277-017-3074-y
[27] Ishikawa Y, Kawashima N, Atsuta Y, et al. Prospective evaluation of prognostic impact of KIT mutations on acute myeloid leukemia with RUNX1-RUNX1T1 and CBFB-MYH11[J]. Blood Adv, 2020, 4(1): 66-75. doi: 10.1182/bloodadvances.2019000709
[28] Schessl C, Rawat VP, Cusan M, et al. The AML1-ETO fusion gene and the FLT3 length mutation collaborate in inducing acute leukemia in mice[J]. J Clin Invest, 2005, 115(8): 2159-2168. doi: 10.1172/JCI24225
[29] Kim HG, Kojima K, Swindle CS, et al. FLT3-ITD cooperates with inv(16) to promote progression to acute myeloid leukemia[J]. Blood, 2008, 111(3): 1567-1574. doi: 10.1182/blood-2006-06-030312
[30] Paschka P, Du J, Schlenk RF, et al. Secondary genetic lesions in acute myeloid leukemia with inv(16) or t(16;16): a study of the German-Austrian AML Study Group(AMLSG)[J]. Blood, 2013, 121(1): 170-177. doi: 10.1182/blood-2012-05-431486
[31] Kayser S, Kramer M, Martínez-Cuadrón D, et al. Characteristics and outcome of patients with core-binding factor acute myeloid leukemia and FLT3-ITD: results from an international collaborative study[J]. Haematologica, 2022, 107(4): 836-843.
-




 下载:
下载: