Early biological markers for early warning of cytokine release syndrome occurrence and severity in relapsed/refractory multiple myeloma treated with CAR-T
-
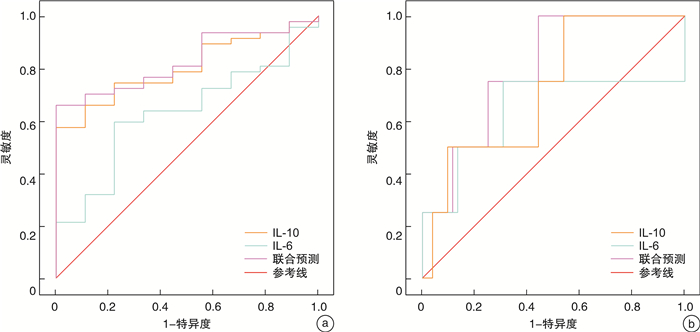
摘要: 目的 探讨嵌合抗原受体T细胞(chimeric antigen receptor T cell,CAR-T)治疗复发/难治性多发性骨髓瘤(relapsed/refractory multiple myeloma, R/R MM)早期生物学标志物对细胞因子释放综合征(cytokine release syndrome,CRS)的发生及严重程度的预警作用。方法 研究纳入2020年10月—2023年1月在徐州医科大学附属医院血液科接受CAR-T细胞治疗,并进行早期生物标志物检测的56例R/R MM患者。分析CAR-T细胞输注后前3天14种生物学标志物浓度水平变化与CRS的发生及严重程度的关系。结果 47例(83.9%)患者发生了CRS,包括43例(76.8%)轻度CRS(1~2级)和4例(7.1%)重度CRS(3~5级)。CRS发生的中位时间为CAR-T细胞输注后第8(4~23)天,中位持续时间是3(1~12) d。细胞输注后早期IL-6(OR=1.018,95%CI 1.000~1.036)和IL-10(OR=1.038,95%CI 1.002~1.074)浓度水平与CRS发生及严重程度密切相关,IL-6与IL-10联合预警CRS发生的灵敏度为65.96%,特异度为100.00%,预警重度(≥3级)CRS发生的灵敏度为100.00%,特异度为55.77%。结论 CAR-T治疗早期,IL-6和IL-10均可以预警CRS的发生,两者联合具有更高的灵敏度。Abstract: Objective To investigate the role of early biological markers of chimeric antigen receptor T cell(CAR-T) therapy for relapsed/refractory multiple myeloma(R/R MM) in early warning of the occurrence and severity of cytokine release syndrome(CRS).Methods Fifty-six patients with R/R MM who were treated with CAR-T cells and underwent early biomarker testing from October 2020 to January 2023 in the Department of Hematology, Affiliated Hospital of Xuzhou Medical University, were enrolled in this study. The relationship between changes in the concentration levels of 14 biological markers and the occurrence and severity of CRS in the first 3 days after CAR-T cell infusion were analyzed.Results CRS occurred in 47(83.9%) patients, including 43(76.8%) with mild CRS(grade 1-2) and 4(7.1%) with severe CRS(grade 3-5). The median time to the onset of CRS was day 8(4-23) after CAR-T cell infusion, and the median duration was 3 days(1-12). The levels of IL-6(OR=1.018, 95%CI 1.000-1.036) and IL-10(OR=1.038, 95%CI 1.002-1.074) concentrations in the early period after cell transfusion were strongly correlated with the occurrence and severity of CRS, and the sensitivity of the combined warning of the occurrence of CRS with IL-6 and IL-10 was 65.96% with a specificity of 100.00%, and the sensitivity of warning the occurrence of severe CRS(≥ grade 3) was 100.00% and the specificity was 55.77%.Conclusion Early in CAR-T therapy, both IL-6 and IL-10 can warn of CRS, and the combination of the two has higher sensitivity.
-

-
表 1 CAR-T输注后CRS的发生和严重程度相关的生物标志物
生物标志物 单因素分析 多因素分析 OR(95%CI) P OR(95%CI) P CRP 0.998(0.982~1.015) 0.857 铁蛋白 1.000(1.000~1.001) 0.216 IL-1β 1.007(0.949~1.069) 0.804 IL-2 1.010(0.992~1.028) 0.290 IL-4 1.046(0.960~1.139) 0.304 IL-5 0.995(0.920~1.075) 0.892 IL-6 1.018(1.002~1.035) 0.029 1.018(1.000~1.036) 0.046 IL-8 1.028(0.993~1.064) 0.116 IL-10 1.037(1.004~1.070) 0.030 1.038(1.002~1.074) 0.036 IL-12P70 1.078(0.821~1.415) 0.589 IL-17 1.035(0.906~1.183) 0.615 IFN-α 1.093(0.887~1.347) 0.403 IFN-γ 1.005(0.964~1.048) 0.805 TNF-α 1.127(0.946~1.344) 0.179 -
[1] Cappell KM, Kochenderfer JN. Long-term outcomes following CAR T cell therapy: what we know so far[J]. Nat Rev Clin Oncol, 2023, 20(6): 359-371. doi: 10.1038/s41571-023-00754-1
[2] 邱婷婷, 王莹, 李德鹏, 等. 多发性骨髓瘤CAR-T治疗后并发噬血细胞综合征1例报道并文献复习[J]. 临床血液学杂志, 2022, 35(11): 805-807. https://lcxy.whuhzzs.com/article/doi/10.13201/j.issn.1004-2806.2022.11.010
[3] Mailankody S, Devlin SM, Landa J, et al. GPRC5D-Targeted CAR T Cells for Myeloma[J]. N Engl J Med, 2022, 387(13): 1196-1206. doi: 10.1056/NEJMoa2209900
[4] Raje N, Berdeja J, Lin Y, et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma[J]. N Engl J Med, 2019, 380(18): 1726-1737. doi: 10.1056/NEJMoa1817226
[5] Wang Y, Cao J, Gu W, et al. Long-Term Follow-Up of Combination of B-Cell Maturation Antigen and CD19 Chimeric Antigen Receptor T Cells in Multiple Myeloma[J]. J Clin Oncol, 2022, 40(20): 2246-2256. doi: 10.1200/JCO.21.01676
[6] Morris EC, Neelapu SS, Giavridis T, et al. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy[J]. Nat Rev Immunol, 2022, 22(2): 85-96. doi: 10.1038/s41577-021-00547-6
[7] Wei Z, Xu J, Zhao C, et al. Prediction of severe CRS and determination of biomarkers in B cell-acute lymphoblastic leukemia treated with CAR-T cells[J]. Front Immunol, 2023, 14: 1273507. doi: 10.3389/fimmu.2023.1273507
[8] Zhou L, Fu W, Wu S, et al. Derivation and validation of a novel score for early prediction of severe CRS after CAR-T therapy in haematological malignancy patients: A multi-centre study[J]. Br J Haematol, 2023, 202(3): 517-524. doi: 10.1111/bjh.18873
[9] Lee DW, Santomasso BD, Locke FL, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells[J]. Biol Blood Marrow Transplant, 2019, 25(4): 625-638. doi: 10.1016/j.bbmt.2018.12.758
[10] Hay KA, Hanafi LA, Li D, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy[J]. Blood, 2017, 130(21): 2295-2306. doi: 10.1182/blood-2017-06-793141
[11] Zhao WH, Liu J, Wang BY, et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma[J]. J Hematol Oncol, 2018, 11(1): 141. doi: 10.1186/s13045-018-0681-6
[12] Parikh RH, Lonial S. Chimeric antigen receptor T-cell therapy in multiple myeloma: A comprehensive review of current data and implications for clinical practice[J]. CA Cancer J Clin, 2023, 73(3): 275-285. doi: 10.3322/caac.21771
[13] 夏洁云, 徐开林. GPRC5D CAR-T细胞治疗复发/难治性多发性骨髓瘤: 单臂Ⅱ期临床试验[J]. 临床血液学杂志, 2023, 36(7): 477-481. https://lcxy.whuhzzs.com/article/doi/10.13201/j.issn.1004-2806.2023.07.005
[14] Schubert ML, Schmitt M, Wang L, et al. Side-effect management of chimeric antigen receptor(CAR)T-cell therapy[J]. Ann Oncol, 2021, 32(1): 34-48. doi: 10.1016/j.annonc.2020.10.478
[15] Kauer J, Hörner S, Osburg L, et al. Tocilizumab, but not dexamethasone, prevents CRS without affecting antitumor activity of bispecific antibodies[J]. J Immunother Cancer, 2020, 8(1): e000621. doi: 10.1136/jitc-2020-000621
[16] Kotch C, Barrett D, Teachey DT. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome[J]. Expert Rev Clin Immunol, 2019, 15(8): 813-822. doi: 10.1080/1744666X.2019.1629904
[17] Norelli M, Camisa B, Barbiera G, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells[J]. Nat Med, 2018, 24(6): 739-748. doi: 10.1038/s41591-018-0036-4
[18] Siglin J, Bukhari A, Lutfi F, et al. C-reactive protein: not always a reliable marker of ongoing cytokine release syndrome in CAR-T therapy following IL-6 blockade[J]. Leuk Lymphoma, 2020, 61(9): 2280-2282. doi: 10.1080/10428194.2020.1757667
[19] Moore KW, de Waal Malefyt R, Coffman RL, et al. Interleukin-10 and the interleukin-10 receptor[J]. Annu Rev Immunol, 2001, 19: 683-765. doi: 10.1146/annurev.immunol.19.1.683
[20] Sabat R, Grütz G, Warszawska K, et al. Biology of interleukin-10[J]. Cytokine Growth Factor Rev, 2010, 21(5): 331-344. doi: 10.1016/j.cytogfr.2010.09.002
[21] Brudno JN, Maric I, Hartman SD, et al. T Cells Genetically Modified to Express an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma[J]. J Clin Oncol, 2018, 36(22): 2267-2280. doi: 10.1200/JCO.2018.77.8084
[22] Gong WJ, Qiu Y, Li MH, et al. Investigation of the risk factors to predict cytokine release syndrome in relapsed or refractory B-cell acute lymphoblastic leukemia patients receiving IL-6 knocking down anti-CD19 chimeric antigen receptor T-cell therapy[J]. Front Immunol, 2022, 13: 922212. doi: 10.3389/fimmu.2022.922212
[23] Kang S, Tanaka T, Inoue H, et al. IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome[J]. Proc Natl Acad Sci U S A, 2020, 117(36): 22351-22356. doi: 10.1073/pnas.2010229117
[24] Teachey DT, Lacey SF, Shaw PA, et al. Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-cell Therapy for Acute Lymphoblastic Leukemia[J]. Cancer Discov, 2016, 6(6): 664-679. doi: 10.1158/2159-8290.CD-16-0040
[25] Lichtenstein DA, Schischlik F, Shao L, et al. Characterization of HLH-like manifestations as a CRS variant in patients receiving CD22 CAR T cells[J]. Blood, 2021, 138(24): 2469-2484. doi: 10.1182/blood.2021011898
-

计量
- 文章访问数: 244
- 施引文献: 0



 下载:
下载: