Safety and efficacy study of mitoxantrone hydrochloride liposome for pre-treatment bridging of CAR-T therapy in lymphoma
-
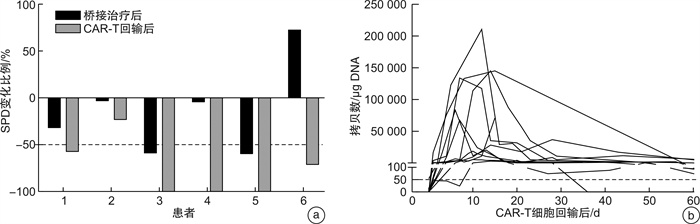
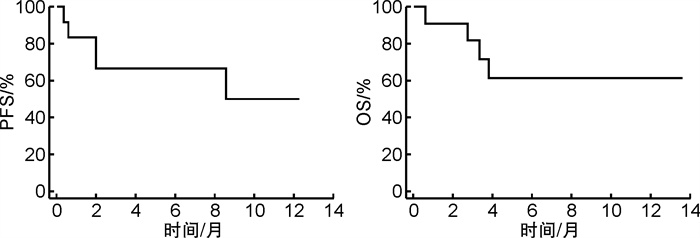
摘要: 目的 目前嵌合抗原受体T(chimeric antigen receptor-T,CAR-T)细胞疗法在复发/难治性淋巴瘤患者的治疗中取得了突破性疗效,桥接治疗作为CAR-T回输前原发病的控制方案与CAR-T疗法的成功输注及后期应答密切相关。本研究旨在通过回顾性研究评估盐酸米托蒽醌脂质体用于CAR-T前桥接方案对于淋巴瘤治疗的疗效及安全性影响,为临床实践方案提供更多选择和依据。方法 回顾性收集和分析2022年3月至2024年4月在我院应用包含盐酸米托蒽醌脂质体作为CAR-T回输前桥接方案的患者的临床资料。参照2014年Lugano修订版的淋巴瘤疗效评价标准、2018年美国移植和细胞治疗协会共识及CTCAE 5.0进行疗效评价和安全性分级;通过即时聚合酶链式反应测定的每微克基因组DNA的转基因拷贝数作为参数评估CAR-T细胞动力学。结果 共有12例复发/难治性淋巴瘤患者纳入研究,中位年龄为48.5(25.0~67.0)岁。9例(75.0%)患者为复发/难治性弥漫大B细胞淋巴瘤,1例为霍奇金淋巴瘤,1例为伯基特淋巴瘤,1例为T淋巴母细胞淋巴瘤。11例(91.7%)患者临床分期为Ⅲ~Ⅳ期;8例(66.7%)患者淋巴瘤IPI评分≥3分;7例(58.3%)患者结外病灶数量≥2个;5例(41.7%)患者既往接受过≥3线治疗;8例(66.7%)患者为大包块/高肿瘤负荷。在CAR-T细胞输注后疗效可评估的11例患者中,总有效率为81.8%(9/11),完全缓解率为54.5%(6/11),部分缓解率为27.3%(3/11)。截至2024年4月30日,1年无进展生存率为50.0%,中位无进展生存期为10.42个月;1年总生存率为61.4%,中位总生存期未达到。CAR-T细胞治疗后,患者均未出现免疫效应细胞相关神经毒性综合征及≥3级细胞因子释放综合征,≥3级的中性粒细胞减少发生率为100.0%,血小板减少发生率为91.7%,贫血发生率为91.7%,绝大多数患者(81.8%)在1个月内恢复到3级以下血液学毒性。结论 盐酸米托蒽醌脂质体用于CAR-T细胞治疗前桥接方案显示出良好的临床应答率,且严重细胞因子释放综合征(≥3级)、免疫效应细胞相关神经毒性综合征发生率低,血液学毒性可控,是针对多种淋巴瘤(弥漫大B细胞淋巴瘤、霍奇金淋巴瘤、伯基特淋巴瘤、T淋母细胞淋巴瘤),特别是具有大包块及高肿瘤负荷患者的CAR-T前桥接治疗的安全有效的选择。
-
关键词:
- 盐酸米托蒽醌脂质体 /
- 淋巴瘤 /
- 桥接治疗 /
- 嵌合抗原受体T细胞治疗
Abstract: Objective Chimeric antigen receptor-T(CAR-T) cell therapy has made a breakthrough in the treatment of patients with relapsed/refractory lymphoma, and bridging therapy as a regimen for controlling primary disease prior to CAR-T therapy is closely related to the successful infusion and treatment response. This study aimed to evaluate the efficacy and safety of using mitoxantrone hydrochloride liposome to bridge to CAR-T in a retrospective study, providing more options and rationale for clinical practice.Methods The clinical data of patients who were treated with bridging therapy containing mitoxantrone hydrochloride liposome for CAR-T infusion from March 2022 to April 2024 were retrospectively collected and analyzed. The efficacy evaluation and safety grading was performed with reference to the revised Lugano 2014 criteria, the 2018 ASTCT consensus and the common terminology criteria for adverse events(CTCAE) version 5.0. The transgene copies per microgram of genomic DNA, determined by real-time quantitative polymerase chain reaction, were used as a parameter to assess CAR-T cellular kinetics.Results A total of 12 patients with relapsed/refractory lymphoma were eventually enrolled in this study. The median age was 48.5(25.0-67.0) years. Nine cases(75.0%) were diagnosed with relapsed/refractory diffuse large B-cell lymphoma, one case was diagnosed Hodgkin lymphoma, one case was diagnosed Burkitt lymphoma, and one case was diagnosed T-lymphoblastic lymphoma. Eleven patients(91.7%) had advanced-stage Ⅲ-Ⅳ; 8 patients(66.7%) had a lymphoma IPI score of more than 3; 7 patients(58.3%) had 2 or more extranodal lesions; and 5 patients(41.7%) had received 3 or more previous treatment lines; 8 patients(66.7%) had a bulky disease(≥7.5 cm) or high tumor burden(MTV>147.5 mL). Among the 11 evaluable patients after CAR-T cell infusion, the objective response rate was 81.8%(9/11), with a complete remission rate of 54.5%(6/11) and a partial remission rate of 27.3%(3/11). Up to April 30, 2024, the 1-year progression-free survival rate was 50.0%, with a median progression-free survival of 10.42 months; the 1-year overall survival rate was 61.4%, with a median overall survival not reached. None of the patients in the cohort suffered from immune effector cell related neurotoxicity syndrome and ≥ grade 3 cytokine release syndrome, and the incidence of ≥ grade 3 hematologic toxicity was 100.0%(neutropenia), 91.7%(thrombocytopenia), and 91.7%(anemia), respectively, while the majority of patients(81.8%) recovered to lower than grade 3 hematologic toxicity within 1 month.Conclusion It indicates that mitoxantrone hydrochloride liposome for bridging to CAR-T therapy shows favorable clinical response and manageable adverse events, suggesting it is a safe and effective option for bridging to CAR-T therapy for a wide range of lymphomas(diffuse large B-cell lymphoma, Hodgkin lymphoma, Burkitt lymphoma, and T-lymphoblastic lymphoma), especially for patients with bulky diseases and/or high tumor burden. -

-
表 1 12例患者的临床基线特征
例(%) 临床特征 患者 年龄/岁 48.5(25.0~67.0) 性别 男 6(50.0) 女 6(50.0) 病理亚型 DLBCL 9(75.0) BL 1(8.3) HL 1(8.3) TLBL 1(8.3) ECOG评分 0~1 6(50.0) 2 6(50.0) 遗传学异常 二次/三次打击重排 1(8.3) TP53缺失/突变 2(16.7) 临床分期 Ⅰ~Ⅱ 1(8.3) Ⅲ~Ⅳ 11(91.7) IPI评分 0~2 4(33.3) 3~5 8(66.7) 结外灶数量≥2个 7(58.3) 大包块(≥7.5 cm) 4(33.3) MTV 高MTV 4(33.3) 低MTV 4(33.3) 不详 4(33.3) 既往治疗线数 中位数 2(1.0~5.0) ≥3线 5(41.7) 既往移植 有 1(8.3) 无 11(91.7) 难治/复发 原发难治 6(50.0) 复发 6(50.0) 表 2 12例患者的治疗方案
例号 桥接方案 免疫治疗方案 CAR-T治疗靶点 1 BiTE+Lipo-MIT CAR-T CD191-1 2 Lipo-MIT ASCT+CRA-T CD19/CD201-3 3 R-MINE ASCT+CRA-T CD19/CD201-3 4 阿糖胞苷+维奈克拉+阿扎胞苷+Lipo-MIT CAR-T CD72 5 Lipo-MIT ASCT+CRA-T CD193 6 BV-MINE ASCT+CRA-T CD304 7 R-MINE CAR-T CD19/CD225 8 R-CMOP CAR-T CD19/CD201-2 9 G+达雷妥尤单抗+地塞米松+Lipo-MIT ASCT+CRA-T CD19/CD201-3 10 G-MINE CAR-T CD191-1 11 MINE ASCT+CRA-T CD19/CD201-3 12 Lipo-MIT CAR-T CD191-1 BiTE:CD3-CD19双特异性抗体;R:利妥昔单抗;MINE:美司钠+异环磷酰胺+Lipo-MIT+依托泊苷;BV:维布妥昔单抗;CMOP:地塞米松+环磷酰胺+长春地辛+Lipo-MIT;G:奥妥珠单抗;ASCT:自体造血干细胞移植。1-1CD19单靶点CAR-T(ClinicalTrials,NCT05388695,队列1);1-3自体造血干细胞移植序贯CD19/CD20双靶点CAR-T(ClinicalTrials,NCT05388695,队列3);2CD7 CAR-T(ClinicalTrials,NCT05398614);3瑞基奥仑赛注射液;4自体造血干细胞移植序贯CD30 CAR-T(ClinicalTrials,ChiCTR2100053662);5CD19/CD22双靶点CAR-T(ClinicalTrials,ChiCTR2000038641);1-2CD19/CD20双靶点CAR-T(ClinicalTrials,NCT05388695,队列2)。 表 3 CAR-T细胞治疗后不良反应发生情况
CAR-T毒性 例数(%) 持续时间/d 持续≤1个月/例(%) CRS 1级 4(33.3) 9.5(2~17) 4(100.0) 2级 8(66.7) 14.5(4~51) 7(87.5) 血液学毒性 ≥3级中性粒细胞减少 12(100.0) 16.5(2~27) 12(100.0) ≥3级血小板减少 11(91.7) 16(3~86) 9(81.8) ≥3级贫血 11(91.7) 22(4~47) 9(81.8) -
[1] June CH, Sadelain M. Chimeric Antigen Receptor Therapy[J]. N Engl J Med, 2018, 379(1): 64-73. doi: 10.1056/NEJMra1706169
[2] Elsallab M, Levine BL, Wayne AS, et al. CAR T-cell product performance in haematological malignancies before and after marketing authorisation[J]. Lancet Oncol, 2020, 21(2): e104-e116. doi: 10.1016/S1470-2045(19)30729-6
[3] Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma[J]. N Engl J Med, 2019, 380(1): 45-56. doi: 10.1056/NEJMoa1804980
[4] Nastoupil LJ, Jain MD, Feng L, et al. Standard-of-Care Axicabtagene Ciloleucel for Relapsed or Refractory Large B-Cell Lymphoma: Results From the US Lymphoma CAR T Consortium[J]. J Clin Oncol, 2020, 38(27): 3119-3128. doi: 10.1200/JCO.19.02104
[5] Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma[J]. N Engl J Med, 2020, 382(14): 1331-1342. doi: 10.1056/NEJMoa1914347
[6] Sesques P, Ferrant E, Safar V, et al. Commercial anti-CD19 CAR T cell therapy for patients with relapsed/refractory aggressive B cell lymphoma in a European center[J]. Am J Hematol, 2020, 95(11): 1324-1333. doi: 10.1002/ajh.25951
[7] Duan Y, You T, Xu P, et al. Effectiveness and Safety of a Cmop±R Regimen for the Initial Treatment of Patients with NHL[C]. 65th ASH Annu Meet Abstr, 2023, 142: 6244.
[8] Wang L, Cao J, Li C, et al. Efficacy and safety of mitoxantrone hydrochloride liposome injection in Chinese patients with advanced breast cancer: a randomized, open-label, active-controlled, single-center, phase Ⅱ clinical trial[J]. Invest New Drugs, 2022, 40(2): 330-339. doi: 10.1007/s10637-021-01182-7
[9] Cai Q, Xia Y, Wang L, et al. Combination of Mitoxantrone Hydrochloride Liposome with Tislelizumab in Patients with Relapsed or Refractory NK/T Cell Lymphoma: A Phase Ib/Ⅱ Clinical Trial[C]. 65th ASH Annu Meet Abstr, 2023, 142: 4470.
[10] Wang X, Ren J, Zhu H, et al. Mitoxantrone Hydrochloride Liposome Injection Combined with Carmustine, Etoposide, and Cytarabine As Conditioning of Autologous Hematopoietic Stem Cell Transplantation in NHL Patients: a Prospective Single-Arm Clinical Trial[C]. 65th ASH Annu Meet Abstr, 2023, 142: 7062.
[11] Song G, Wu H, Yoshino K, et al. Factors affecting the pharmacokinetics and pharmacodynamics of liposomal drugs[J]. J Liposome Res, 2012, 22(3): 177-192. doi: 10.3109/08982104.2012.655285
[12] Zhang H, Sheng D, Han Z, et al. Doxorubicin-liposome combined with clodronate-liposome inhibits hepatocellular carcinoma through the depletion of macrophages and tumor cells[J]. Int J Pharm, 2022, 629: 122346. doi: 10.1016/j.ijpharm.2022.122346
[13] 赵倩, 王彩霞, 邱云良, 等. 盐酸米托蒽醌脂质体的药效学及毒性研究[J]. 中国药理学通报, 2011, 27(12): 4-4. https://www.cnki.com.cn/Article/CJFDTOTAL-YAOL201112028.htm
[14] Khoury JD, Solary E, Abla O, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms[J]. Leukemia, 2022, 36(7): 1703-1719. doi: 10.1038/s41375-022-01613-1
[15] Dean EA, Mhaskar RS, Lu H, et al. High metabolic tumor volume is associated with decreased efficacy of axicabtagene ciloleucel in large B-cell lymphoma[J]. Blood Adv, 2020, 4(14): 3268-3276. doi: 10.1182/bloodadvances.2020001900
[16] Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification[J]. J Clin Oncol, 2014, 32(27): 3059-3068. doi: 10.1200/JCO.2013.54.8800
[17] Lee DW, Santomasso BD, Locke FL, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells[J]. Biol Blood Marrow Transplant, 2019, 25(4): 625-638. doi: 10.1016/j.bbmt.2018.12.758
[18] Hines MR, Knight TE, McNerney KO, et al. Immune Effector Cell-Associated Hemophagocytic Lymphohistiocytosis-Like Syndrome[J]. Transplant Cell Ther, 2023, 29(7): 438. e1-438. e16. doi: 10.1016/j.jtct.2023.03.006
[19] Iacoboni G, Simó M, Villacampa G, et al. Prognostic impact of total metabolic tumor volume in large B-cell lymphoma patients receiving CAR T-cell therapy[J]. Ann Hematol, 2021, 100(9): 2303-2310. doi: 10.1007/s00277-021-04560-6
[20] Zhou L, Yu N, Li T, et al. Clinical characteristics and prognosis of 16 relapsed/refractory B-cell malignancy patients with CAR T-cell-related hyperferritinaemia[J]. Front Oncol, 2022, 12: 912689. doi: 10.3389/fonc.2022.912689
[21] Hayden PJ, Roddie C, Bader P, et al. Management of adults and children receiving CAR T-cell therapy: 2021 best practice recommendations of the European Society for Blood and Marrow Transplantation(EBMT)and the Joint Accreditation Committee of ISCT and EBMT(JACIE)and the European Haematology Association(EHA)[J]. Ann Oncol, 2022, 33(3): 259-275. doi: 10.1016/j.annonc.2021.12.003
[22] Freyer CW, Porter DL. Cytokine release syndrome and neurotoxicity following CAR T-cell therapy for hematologic malignancies[J]. J Allergy Clin Immuno, 2020, 146(5): 940-948. doi: 10.1016/j.jaci.2020.07.025
[23] Pinnix CC, Gunther JR, Dabaja BS, et al. Bridging therapy prior to axicabtagene ciloleucel for relapsed/refractory large B-cell lymphoma[J]. Blood Adv, 2020, 4(13): 2871-2883. doi: 10.1182/bloodadvances.2020001837
-




 下载:
下载: