Efficacy and safety of orelabrutinib-based regimens in 28 cases of newly diagnosed diffuse large B-cell lymphoma
-
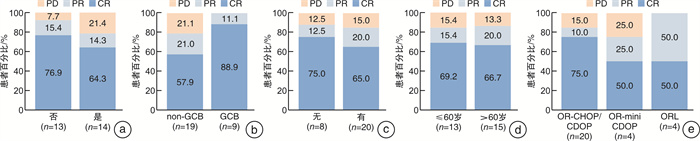
摘要: 目的 探讨基于奥布替尼的联合方案治疗初治弥漫性大B细胞淋巴瘤(diffuse large B-cell lymphoma,DLBCL)患者的疗效和安全性。方法 回顾性分析2020年9月至2023年10月在华中科技大学同济医学院附属协和医院血液科接受基于奥布替尼联合方案治疗的初治DLBCL患者的临床资料。结果 共纳入28例患者,中位年龄61.5(36~83)岁。双表达淋巴瘤14例;老年(年龄>60岁)患者15例。所有患者中20例使用奥布替尼联合R-CHOP/CDOP方案,4例使用奥布替尼联合R-miniCDOP方案,4例使用奥布替尼联合利妥昔单抗和来那度胺(ORL)方案。使用PET/CT评估疗效,所有患者的客观缓解率(objective response rate,ORR)为85.7%,完全缓解(complete remission,CR)率为67.9%,疾病进展(progressive disease,PD)率为14.3%。双表达患者的ORR为78.6%,CR率为64.3%,PD率为21.4%。年龄>60岁患者的ORR为86.7%,CR率为66.7%,PD率为13.3%。使用奥布替尼联合R-CHOP/CDOP方案患者的ORR为85.0%,CR率为75.0%,PD率为15.0%;使用奥布替尼联合R-miniCDOP方案患者的ORR为75.0%,CR率为50.0%,PD率为25.0%;使用ORL方案患者的ORR为100.0%,CR率为50.0%。中位随访14.5(4~40)个月,所有患者的预期3年总生存(overall survival,OS)率和无进展生存(progression-free survival,PFS)率分别为84.9%和70.6%,双表达患者的预期3年OS率和PFS率分别为100.0%和70.9%,老年患者的预期3年OS率和PFS率分别为71.4%和65.5%。双表达和非双表达患者的ORR、CR率、预期3年OS率和预期3年PFS率比较均差异无统计学意义。老年和年轻患者的ORR、CR率、预期3年OS率和预期3年PFS率比较差异亦无统计学意义。最常见的不良反应为贫血(1~2级76.9%、3~4级19.2%)、中性粒细胞减少(1~2级46.2%、3~4级38.5%)及血小板减少(1~2级34.6%、3~4级15.4%);最常见的非血液学不良反应为肺部感染(17.9%)、食欲减退(10.7%)、恶心(10.7%)和呕吐(10.7%)。结论 基于奥布替尼的联合方案在初治DLBCL患者中展现出良好的疗效和可控的安全性,可能改善双表达淋巴瘤和老年患者的疗效及预后,值得进一步探索。
-
关键词:
- 弥漫性大B细胞淋巴瘤 /
- 奥布替尼
Abstract: Objective To investigate the efficacy and safety of orelabrutinib-based regimens in newly diagnosed diffuse large B-cell lymphoma(DLBCL).Methods The clinical data of 28 newly diagnosed patients with DLBCL, who were treated with orelabrutinib-based regimens between September 2020 and October 2024 in the Institute of Hematology in Union Hospital of Tongji Medical College of Huazhong University of Science and Technology, were collected and retrospectively analyzed.Results A total of 28 patients with a median age of 61.5 years(range 36-83 years) were included. Fourteen patients had double-expression lymphoma(DEL) and 15 patients were 60 years old and above. Of all the patients, 20 cases received orelabrutinib in combination with R-CHOP/CDOP(OR-CHOP/CDOP) regimens, 4 cases received orelabrutinib in combination with R-miniCDOP(OR-miniCDOP) regimens, and 4 cases received ORL regimens. PET/CT was used for the evaluation of the treatment response. The objective response rate(ORR) and complete remission(CR) rate of all the patients were 85.7% and 67.9%, and the progressive disease(PD) rate was 14.3%. The ORR, CR rate and PD rate of the DEL patients were 78.6%, 64.3% and 21.4%, respectively. The ORR, CR rate and PD rate of the elderly patients(>60 years) were 86.7%, 66.7% and 13.3%, respectively. The ORR, CR rate and PD rate were 85.0%, 75.0% and 15.0% in the OR-CHOP/CDOP regimens; the ORR, CR rate and PD rate were 75.0%, 50.0% and 25.0% in the OR-miniCDOP regimens; the ORR and CR rate were 100.0% and 50.0% in the ORL regimens. With a median follow-up of 14.5 months, the estimated 3-year overall survival(OS) and progression-free survival(PFS) rate were 84.9% and 70.6% for all the patients, 100.0% and 70.9% for the DEL patients, and 71.4% and 65.5% for the elderly patients. There was no statistical difference in ORR, CR rate, 3-year OS and PFS between the DEL and non-DEL patients. There was no statistical difference in ORR, CR rate, 3-year OS and PFS between the elderly and young patients, neither. The most common adverse events were anemia(76.9% of grade 1-2, 19.2% of grade 3-4), neutropenia(46.2% of grade 1-2, 38.5% of grade 3-4) and thrombocytopenia(34.6% of grade 1-2, 15.4% of grade 3-4). The most common non-hematological adverse events were pulmonary infection(17.9%), loss of appetite(10.7%), nausea(10.7%) and vomiting(10.7%).Conclusion Orelabrutinib-based regimens show promising efficacy and tolerable safety in newly diagnosed DLBCL, with a promising improvement of the efficacy and survival in the DEL and old patients, which deserves further study.-
Key words:
- diffuse large B-cell lymphoma /
- orelabrutinib
-

-
表 1 DLBCL患者基本情况
例(%) 特征 整个队列 患者例数 28 中位年龄/岁 61.5(36~83) 性别 男 15(53.6) 女 13(46.4) ECOG评分 0~1 23(82.1) ≥2 5(17.9) B症状 无 19(67.9) 有 9(32.1) 诊断时乳酸脱氢酶 正常 16(57.1) 升高 12(42.9) Lugano分期 Ⅰ~Ⅱ期 13(46.4) Ⅲ~Ⅳ期 15(53.6) IPI评分 0~2 18(64.3) 3~5 10(35.7) 细胞起源 GCB 9(32.1) non-GCB 19(67.9) DEL 否 13(46.4) 是 14(50.0) 未知 1(3.6) 骨髓受累 无 25(89.3) 有 3(10.7) 结外累及 无 8(28.6) 有 20(71.4) 结外病灶数/个 <2 10(50.0) ≥2 10(50.0) 中位随访时间/月 14.5(4~40) 中位PFS时间/月 14.5(4~40) ECOG评分:美国东部肿瘤协作组活动状态评分;IPI:国际预后指数。 表 2 患者治疗相关不良反应情况
例(%) 整个队列 1~2级 3~4级 5级 血液学不良反应△ 贫血 20(76.9) 5(19.2) 血小板减少 9(34.6) 4(15.4) 中性粒细胞减少 12(46.2) 10(38.5) 发热性中性粒细胞减少 9(34.6) 紫癜 2(7.1) 非血液学不良反应 肺部感染 5(17.9) 颅内感染 1(3.6) 心律失常 2(7.1) 心包积液 1(3.6) 肝功能不良 2(7.1) 食欲减退 3(10.7) 恶心 3(10.7) 呕吐 3(10.7) △血液学不良反应中部分患者数据缺失。 -
[1] Geng H, Jia S, Zhang Y, et al. Efficacy and safety of zanubrutinib plus R-CHOP in treatment of non-GCB DLBCL with extranodal involvement[J]. Front Immunol, 2023, 14: 1219167. doi: 10.3389/fimmu.2023.1219167
[2] Liu Y, Barta SK. Diffuse Large B-Cell Lymphoma: 2019 Update on Diagnosis, Risk Stratification, and Treatment[J]. Am J Hematol, 2019, 94(5): 604-616. doi: 10.1002/ajh.25460
[3] Poletto S, Novo M, Paruzzo L, et al. Treatment Strategies for Patients with Diffuse Large B-Cell Lymphoma[J]. Cancer Treat Rev, 2022, 110: 102443. doi: 10.1016/j.ctrv.2022.102443
[4] 吴剑秋, 刘艳艳, 李菲, 等. Polatuzumab vedotin联合方案治疗复发难治性弥漫大B细胞淋巴瘤的有效性及安全性研究[J]. 中华医学杂志, 2021, 101(25): 1985-1990. doi: 10.3760/cma.j.cn112137-20201030-02971
[5] 中国临床肿瘤学会(CSCO)淋巴瘤专家委员会. 布鲁顿酪氨酸激酶抑制剂治疗B细胞恶性肿瘤中国专家共识[J]. 白血病·淋巴瘤, 2022, 31(9): 513-526. doi: 10.3760/cma.j.cn115356-20220718-00211
[6] Alu A, Lei H, Han X, et al. BTK inhibitors in the treatment of hematological malignancies and inflammatory diseases: mechanisms and clinical studies[J]. J Hematol Oncol, 2022, 15(1): 138. doi: 10.1186/s13045-022-01353-w
[7] Robak P, Witkowska M, Wolska-Washer A, et al. The preclinical discovery and development of orelabrutinib as a novel treatment option for B-cell lymphoid malignancies[J]. Expert Opin Drug Discov, 2023, 18(10): 1065-1076. doi: 10.1080/17460441.2023.2236547
[8] Xu PP, Liu T, Li Z, et al. Efficacy and safety of orelabrutinib in diffuse large B-cell lymphoma: A real-world analysis[J]. JCO, 2022, 40(16_suppl): e19556-e19556. doi: 10.1200/JCO.2022.40.16_suppl.e19556
[9] Tilly H, Morschhauser F, Sehn LH, et al. Polatuzumab Vedotin in Previously Untreated Diffuse Large B-Cell Lymphoma[J]. N Engl J Med, 2022, 386(4): 351-363. doi: 10.1056/NEJMoa2115304
[10] Vodicka P, Klener P, Trneny M. Diffuse Large B-Cell Lymphoma(DLBCL): Early Patient Management and Emerging Treatment Options[J]. Onco Targets Ther, 2022, 15: 1481-1501. doi: 10.2147/OTT.S326632
[11] Yang Y, He J, Zhao Y, et al. Preliminary Outcomes of Orelabrutinib Plus RCHOP in Treatment-Naive Patients with Double-Expression Diffuse Large B Cell Lymphoma[J]. Blood, 2022, 140(Supplement 1): 12102-12103. doi: 10.1182/blood-2022-168017
[12] Deng T, Zhang S, Xiao M, et al. A single-centre, real-world study of BTK inhibitors for the initial treatment of MYD88mut/CD79Bmut diffuse large B-cell lymphoma[J]. Cancer Med, 2024, 13(4): e7005. doi: 10.1002/cam4.7005
[13] Shi Y, Han Y, Yang J, et al. Clinical features and outcomes of diffuse large B-cell lymphoma based on nodal or extranodal primary sites of origin: Analysis of 1, 085 WHO classified cases in a single institution in China[J]. Chin J Cancer Res, 2019, 31(1): 152-161. doi: 10.21147/j.issn.1000-9604.2019.01.10
[14] Sehn LH, Salles G. Diffuse Large B-Cell Lymphoma[J]. N Engl J Med, 2021, 384(9): 842-858. doi: 10.1056/NEJMra2027612
[15] Younes A, Sehn LH, Johnson P, et al. Randomized Phase Ⅲ Trial of Ibrutinib and Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Non-Germinal Center B-Cell Diffuse Large B-Cell Lymphoma[J]. J Clin Oncol, 2019, 37(15): 1285-1295. doi: 10.1200/JCO.18.02403
[16] Yu H, Wang X, Li J, et al. Addition of BTK inhibitor orelabrutinib to rituximab improved anti-tumor effects in B cell lymphoma[J]. Mole Ther Oncolytics, 2021, 21: 158-170. doi: 10.1016/j.omto.2021.03.015
[17] Xu PP, Shi ZY, Qian Y, et al. Ibrutinib, rituximab, and lenalidomide in unfit or frail patients aged 75 years or older with de novo diffuse large B-cell lymphoma: a phase 2, single-arm study[J]. Lancet Healthy Longevity, 2022, 3(7): e481-e490. doi: 10.1016/S2666-7568(22)00123-4
[18] Shirley M. Bruton Tyrosine Kinase Inhibitors in B-Cell Malignancies: Their Use and Differential Features[J]. Target Oncol, 2022, 17(1): 69-84. doi: 10.1007/s11523-021-00857-8
[19] Song Y, Xu W, Song Y, et al. Pooled Analysis of Safety Data from Clinical Trials of Orelabrutinib Monotherapy in Hematologic Malignancies[J]. Blood, 2020, 136(Supplement 1): 43-43.
[20] 中国临床肿瘤学会(CSCO)淋巴瘤专家委员会. 奥布替尼治疗B细胞淋巴瘤中国专家推荐临床应用指导原则(2021年版)[J]. 白血病·淋巴瘤, 2021, 30(8): 455-460. doi: 10.3760/cma.j.cn115356-20210723-00162
-




 下载:
下载: