Safety and efficacy of allogeneic donor-derived CD19 CAR-T therapy for the treatment of relapsed B-cell acute lymphoblastic leukemia after transplantation
-
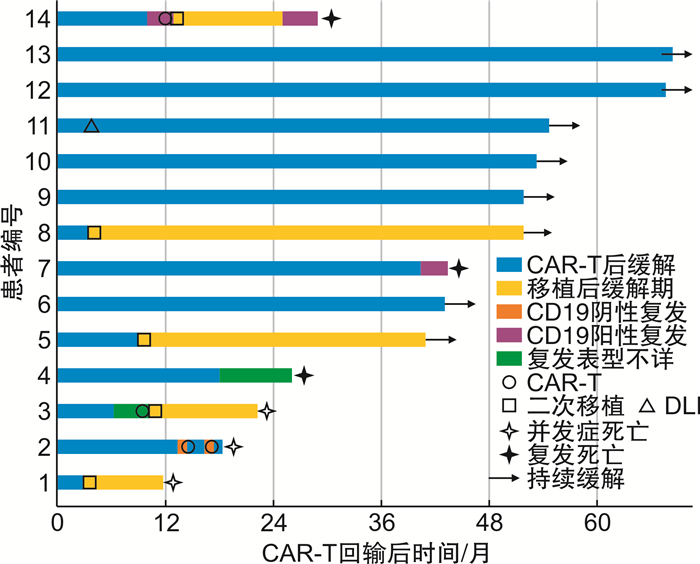
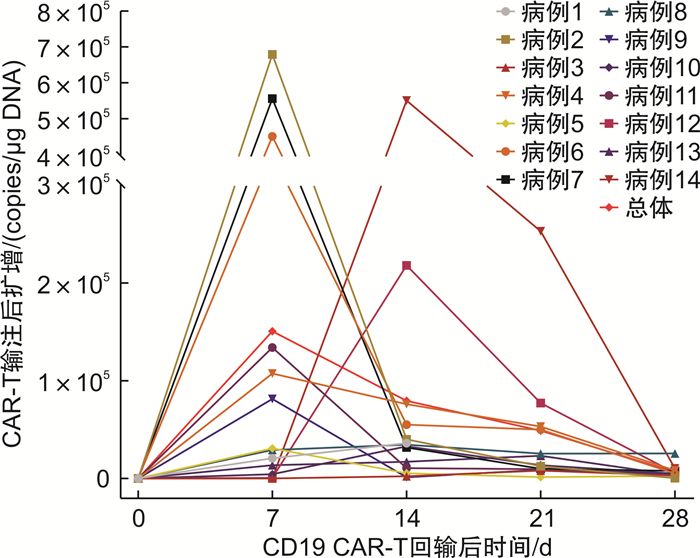
摘要: 目的 探讨供者CD19嵌合抗原受体T细胞(chimeric antigen receptor T cell,CAR-T)治疗在儿童急性B淋巴细胞白血病(B-cell acute lymphoblastic leukemia,B-ALL)接受异基因造血干细胞移植后复发患者中的疗效及安全性。方法 选择2018年1月至2020年12月我中心收治的14例采用供者CD19 CAR-T细胞治疗的移植后复发B-ALL的临床资料,评估其疗效及安全性。结果 14例患者在回输后1个月内均获得骨髓微小残留病阴性的完全缓解。所有患者均发生细胞因子释放综合征,严重细胞因子释放综合征(≥3级)者9例(64.3%);4例发生免疫效应细胞相关神经毒性综合征,均经相应治疗后症状消失。中位随访时间为43.4(11.8~ 68.4)个月,5例复发,其中4例为移植后骨髓血液学复发患者,1例为移植后微小残留病水平复发患者,复发时CD19阳性2例,CD19阴性1例,另外2例表型不详,中位复发时间为13.4个月,3年总生存率为63%±13%,3年无白血病生存率为61%±14%。结论 供者CD19 CAR-T细胞治疗可有效治疗移植后复发B-ALL,但对回输前肿瘤负荷高的患者,建议再次缓解后桥接造血干细胞移植以改善长期生存情况。
-
关键词:
- 供者嵌合抗原受体 /
- CD19 /
- 异基因造血干细胞移植 /
- 急性B淋巴细胞白血病 /
- 复发
Abstract: Objective To investigate the efficacy and safety of donor CD19 chimeric antigen receptor T cell(CAR-T) therapy in pediatric patients with B-cell acute lymphoblastic leukemia(B-ALL) who relapsed after allogeneic hematopoietic stem cell transplantation.Methods The clinical data of 14 patients with post-transplant relapsed B-ALL treated with donor CD19 CAR-T cell therapy were collected from January 2018 to December 2020, and the efficacy and safety of CAR-T cell therapy were evaluated.Results All of the 14 patients obtained negative minimal residual disease within one month after infusion. Cytokine release syndrome occurred in all patients, with 9 patients(64.3%) developed severe cytokine release syndrome(grade ≥3), and 4 patients developed immune effector cell-associated neurotoxicity syndrome, all of which disappeared after corresponding treatment. The median follow-up time was 43.4(11.8-68.4) months, and there were 5 cases of recurrence with a median recurrence time of 13.4 months. Four cases were identified as bone marrow relapse prior to CAR-T cell therapy, and only one case was minimal residual disease recurrence. At relapse, the CD19 immunophenotype on leukemia blasts was CD19+(2 cases), CD19-(1 case), and CD19 unknown(2 cases). The 3-year overall survival rate was 63%±13%, and the 3-year leukemia-free survival rate was 61%±14%.Conclusion Donor CD19 CAR-T cell therapy can effectively treat recurrent B-ALL after transplantation, but for patients with high tumor burden before infusion, it is recommended to bridge to hematopoietic stem cell transplantation after remission to improve long-term survival. -

-
表 1 14例allo-HSCT后复发B-ALL患者的临床资料及接受CD19 CAR-T细胞治疗的疗效
例号 移植前疾病状态 遗传学异常 移植供者类型 移植后复发类型 预处理前骨髓形态缓解情况(原幼细胞比例) CAR-T后是否复发 预后 转归 LFS时间/月 OS时间/月 1 CR1 TCF3/PBX1 MSD MRD CR 否 11.8 11.8 死于二次移植后肺部排异 2 CR1 无 单倍型 血液学复发 47% 是 13.4 19.4 死于CD22 CAR-T后严重CRS 3 CR2 超二倍体 MSD 血液学复发 16% 是 6.3 20.8 死于二次移植后肺部排异 4 CR1 超二倍体 单倍型 MRD CR 是 18.1 26.3 死于骨髓复发 5 CR1 MLL-ENL阳性 单倍型 MRD CR 否 40.8 40.8 CR 6 CR1 无 单倍型 血液学复发 55.5% 否 43.1 43.1 CR 7 CR1 无 MSD 血液学复发 5% 是 40.4 43.4 死于骨髓复发 8 CR1 无 单倍型 血液学复发 49% 否 51.7 51.7 CR 9 CR1 MLL FISH重排阳性 MSD MRD CR 否 51.8 51.8 CR 10 CR1 MLL-AF1q阳性 单倍型 MRD CR 否 53.2 53.2 CR 11 CR2 MLL-AF10阳性 单倍型 MRD+EMR CR 否 54.6 54.6 CR 12 CR2 无 单倍型 血液学复发 8% 否 67.7 67.7 CR 13 CR2 TCF3/PBX1 单倍型 MRD CR 否 68.4 68.4 CR 14 CR2 IKZF1阳性,超二倍体 单倍型 血液学复发 88% 是 10.3 48.5 死于骨髓复发 -
[1] Nagler A, Labopin M, Houhou M, et al. Outcome of haploidentical versus matched sibling donors in hematopoietic stem cell transplantation for adult patients with acute lymphoblastic leukemia: a study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation[J]. J Hematol Oncol, 2021, 14(1): 53. doi: 10.1186/s13045-021-01065-7
[2] Pavlu J, Labopin M, Niittyvuopio R, et al. Measurable residual disease at myeloablative allogeneic transplantation in adults with acute lymphoblastic leukemia: a retrospective registry study on 2780 patients from the acute leukemia working party of the EBMT[J]. J Hematol Oncol, 2019, 12(1): 108. doi: 10.1186/s13045-019-0790-x
[3] 姜尔烈, 郑亚伟. 靶向治疗及免疫治疗时代造血干细胞移植在急性白血病中的应用[J]. 临床血液学杂志, 2023, 36(9): 615-621. https://lcxy.whuhzzs.com/article/doi/10.13201/j.issn.1004-2806.2023.09.002
[4] Wolach O, Amitai I, DeAngelo DJ. Current challenges and opportunities in treating adult subjects with Philadelphia-negative acute lymphoblastic leukaemia[J]. Br J Haematol, 2017, 179(5): 705-723. doi: 10.1111/bjh.14916
[5] 王雨馨, 李玉华. 高危白血病移植后复发行供者淋巴细胞输注治疗的疗效分析[J]. 中国实验血液学杂志, 2015, 23(4): 982-988.
[6] Li Z, Yang K, Song Y, et al. CAR-T therapy followed by allogeneic hematopoietic stem cell transplantation for refractory/relapsed acute B lymphocytic leukemia: Long-term follow-up results[J]. Front Oncol, 2023, 12: 1048296. doi: 10.3389/fonc.2022.1048296
[7] Zhang X, Yang J, Li J, et al. Factors associated with treatment response to CD19 CAR-T therapy among a large cohort of B cell acute lymphoblastic leukemia[J]. Cancer Immunol Immunother, 2022, 71(3): 689-703. doi: 10.1007/s00262-021-03009-z
[8] An F, Wang H, Liu Z, et al. Influence of patient characteristics on chimeric antigen receptor T cell therapy in B-cell acute lymphoblastic leukemia[J]. Nat Commun, 2020, 11(1): 5928. doi: 10.1038/s41467-020-19774-x
[9] Sidaway P. Allogeneic CAR T cells show promise[J]. Nat Rev Clin Oncol, 2022, 19(12): 748.
[10] Qasim W. Allogeneic CAR T cell therapies forleukemia[J]. Am J Hematol, 2019, 94(S1): S50-S54.
[11] Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells[J]. Biol Blood Marrow Transplant, 2019, 25(4): 625-638. doi: 10.1016/j.bbmt.2018.12.758
[12] Zhang X, Lu XA, Yang J, et al. Efficacy and safety of anti-CD19 CAR T-cell therapy in 110 patients with B-cell acute lymphoblastic leukemia with high-risk features[J]. Blood Adv, 2020, 4(10): 2325-2338. doi: 10.1182/bloodadvances.2020001466
[13] Chen YH, Zhang X, Cheng YF, et al. Long-term follow-up of CD19 chimeric antigen receptor T-cell therapy for relapsed/refractory acute lymphoblastic leukemia after allogeneic hematopoietic stem cell transplantation[J]. Cytotherapy, 2020, 22(12): 755-761. doi: 10.1016/j.jcyt.2020.08.002
[14] Ding L, Wang Y, Hong R, et al. Efficacy and safety of chimeric antigen receptor T cells in acute lymphoblastic leukemia with post-transplant relapse[J]. Front Oncol, 2021, 11: 750218. doi: 10.3389/fonc.2021.750218
[15] Zhang C, Wang XQ, Zhang RL, et al. Donor-derived CD19 CAR-T cell therapy of relapse of CD19-positive B-ALL post allotransplant[J]. Leukemia, 2021, 35(6): 1563-1570. doi: 10.1038/s41375-020-01056-6
[16] Cao XY, Zhang JP, Zhao YL, et al. Analysis benefits of a second Allo-HSCT after CAR-T cell therapy in patients with relapsed/refractory B-cell acute lymphoblastic leukemia who relapsed after transplant[J]. Front Immunol, 2023, 14: 1191382. doi: 10.3389/fimmu.2023.1191382
[17] Shah NN, Lee DW, Yates B, et al. Long-Term Follow-Up of CD19-CAR T-Cell therapy in children and young adults with B-ALL[J]. J Clin Oncol, 2021, 39(15): 1650-1659. doi: 10.1200/JCO.20.02262
[18] Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma[J]. N Engl J Med, 2019, 380(1): 45-56. doi: 10.1056/NEJMoa1804980
[19] Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas(TRANSCEND NHL 001): a multicentre seamless design study[J]. Lancet, 2020, 396(10254): 839-852. doi: 10.1016/S0140-6736(20)31366-0
[20] Frey NV, Gill S, Hexner EO, et al. Long-Term Outcomes From a Randomized Dose Optimization Study of Chimeric Antigen Receptor Modified T Cells in Relapsed Chronic Lymphocytic Leukemia[J]. J Clin Oncol, 2020, 38(25): 2862-2871. doi: 10.1200/JCO.19.03237
[21] Chou CK, Turtle CJ. Assessment and management of cytokine release syndrome and neurotoxicity following CD19 CAR-T cell therapy[J]. Expert Opin Biol Ther, 2020, 20(6): 653-664. doi: 10.1080/14712598.2020.1729735
[22] Zhang X, Yang J, Li J, et al. Factors associated with treatment response to CD19 CAR-T therapy among a large cohort of B cell acute lymphoblastic leukemia[J]. Cancer Immunol Immunother, 2022, 71(3): 689-703. doi: 10.1007/s00262-021-03009-z
[23] Hu L, Charwudzi A, Li Q, et al. Anti-CD19 CAR-T cell therapy bridge to HSCT decreases the relapse rate and improves the long-term survival of R/R B-ALL patients: a systematic review and meta-analysis[J]. Ann Hematol, 2021, 100(4): 1003-1012. doi: 10.1007/s00277-021-04451-w
-

计量
- 文章访问数: 325
- 施引文献: 0



 下载:
下载: