A retrospective cohort study of hetrombopag combined with recombinant human thrombopoietin in the treatment of thrombocytopenia due to antineoplastic therapy
-
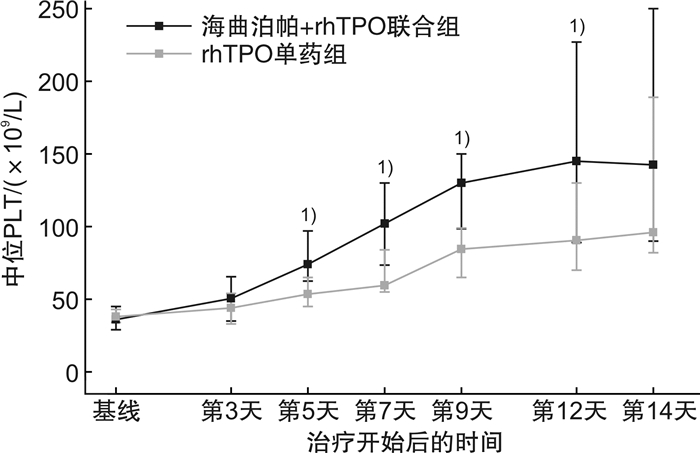
摘要: 目的 回顾性分析TPO受体激动剂(TPO-RA)+重组人血小板生成素(rhTPO)联合给药与rhTPO单药治疗肿瘤治疗所致血小板减少症(cancer therapy induced thrombocytopenia,CTIT)的有效性和安全性,探索联合给药的临床益处,为CTIT患者带来新的治疗选择。方法 回顾性分析应用海曲泊帕联合rhTPO与rhTPO单药治疗CTIT患者的临床资料。主要研究终点为7 d内治疗应答比例。结果 本研究共纳入58例符合纳入与排除标准的CTIT患者,其中海曲泊帕+rhTPO联合组28例,rhTPO单药组30例。海曲泊帕+rhTPO联合组7 d内治疗应答比例为75%(21/28),rhTPO单药组为30%(9/30),差异有统计学意义(P<0.05)。海曲泊帕+rhTPO联合组中位治疗天数为6.5 d,rhTPO单药组中位治疗天数为9.5 d,差异有统计学意义(P<0.05)。结论 研究表明海曲泊帕联合rhTPO给药可能为3/4级血小板减少症患者提供一种新的治疗选择,可以更快、更有效地提高血小板水平,且安全性良好。
-
关键词:
- 海曲泊帕 /
- 重组人血小板生成素 /
- 肿瘤治疗所致血小板减少症 /
- 血小板生成素
Abstract: Objective To retrospectively analyze the effectiveness and safety of TPO-RA in combination with rhTPO vs rhTPO alone, and to explore the benefits of the combination therapy in order to bring new treatment options for patients with cancer therapy induced thrombocytopenia(CTIT).Methods The clinical data of CTIT patients who received hetrombopag in combination with rhTPO vs. rhTPO alone were retrospectively analyzed. The primary outcome was the proportion of treatment response within 7 days.Results A total of 58 patients with CTIT who met the case screening criteria were included in this study, including 28 patients in the hetrombopag+rhTPO group and 30 patients in the rhTPO alone group. The proportion of treatment response within 7 days was 75%(21/28) in the hetrombopag+rhTPO group and 30%(9/30) in the rhTPO monotherapy group. There were significant difference between the two groups(P < 0.05). The median treatment duration were 6.5 days in the hetrombopag+rhTPO group and 9.5 days in the rhTPO monotherapy group. There were significant difference between the two groups(P < 0.05).Conclusion This retrospective study suggests that hetrombopag combined with rhTPO may be a treatment option for patients with grade 3/4 thrombocytopenia by providing a faster and more effective boost in platelet levels without increasing safety concerns.-
Key words:
- hetrombopag /
- rhTPO /
- cancer therapy induced thrombocytopenia /
- thrombopoietin
-

-
表 1 2组患者基线特征
基线特征 海曲泊帕+rhTPO联合组(n=28) rhTPO单药组(n=30) 中位年龄/岁 65.5(30.0~79.0) 65.5(30.0~79.0) 年龄/例(%) 18~65岁 14(50.0) 15(50.0) >65岁 14(50.0) 15(50.0) 中位体重/kg 60(43~88) 60(43~80) 性别/例(%) 男 15(53.6) 15(50.0) 女 13(46.4) 15(50.0) 肿瘤类型/例(%) 肺癌 4(14.3) 8(26.7) 胃肠道肿瘤 16(57.1) 10(33.3) 妇科肿瘤 6(21.4) 4(13.3) 其他 2(7.1) 8(26.7) 肿瘤分期/例(%) Ⅱ期 2(7.1) 2(6.7) Ⅲ期 7(25.0) 6(20.0) Ⅳ期 19(67.9) 22(73.3) ECOG评分/例(%) 0 12(42.9) 11(36.7) 1 10(35.7) 14(46.7) 2 6(21.4) 5(16.7) 中位PLT/(×109/L) 36(5~49) 38(16~47) PLT/例(%) <30×109/L 8(28.6) 3(10.0) 30×109/L~50×109/L 20(71.4) 27(90.0) 表 2 2组患者7 d和14 d内疗效反应
组别 例数 7 d内疗效评价 14 d内疗效评价 PLT升高≥50×109/L患者比例 PLT升高至少1倍患者比例 PLT升至≥100×109/L患者比例 PLT升高≥50×109/L患者比例 PLT升高至少1倍患者比例 PLT升至≥100×109/L患者比例 海曲泊帕+rhTPO联合组 28 67.9%(19/28) 85.7%(24/28) 50.0%(14/28) 96.4%(27/28) 100.0%(28/28) 75.0%(21/28) rhTPO单药组 30 16.7%(5/30) 33.3%(10/30) 10.0%(3/30) 63.3%(19/30) 80.0%(24/30) 53.3%(16/30) P <0.00 01 0.000 2 0.002 2 0.005 4 0.024 0 0.086 2 -
[1] 中国临床肿瘤学会指南工作委员会. 中国临床肿瘤学会(CSCO)肿瘤治疗所致血小板减少症诊疗指南2022[M]. 北京: 人民卫生出版社, 2022.
[2] Elting LS, Rubenstein EB, Martin CG, et al. Incidence, cost, and outcomes of bleeding and chemotherapy dose modification among solid tumor patients with chemotherapy-induced thrombocytopenia[J]. J Clin Oncol, 2001, 19(4): 1137-1146. doi: 10.1200/JCO.2001.19.4.1137
[3] Avvisati G, Tirindelli MC, Annibali O. Thrombocytopenia and hemorrhagic risk in cancer patients[J]. Crit Rev Oncol Hematol, 2003, 48(Suppl): S13-S16.
[4] Wu Y, Aravind S, Ranganathan G, et al. Anemia and thrombocytopenia in patients undergoing chemotherapy for solid tumors: a descriptive study of a large outpatient oncology practice database, 2000-2007[J]. Clin Ther, 2009, 31(Pt 2): 2416-2432.
[5] 王理伟. 《肿瘤治疗相关血小板减少症的临床管理专家共识》述评[J]. 肿瘤, 2021, 41(12): 828-831.
[6] 中国抗癌协会肿瘤临床化疗专业委员会, 中国抗癌协会肿瘤支持治疗专业委员会. 中国肿瘤化疗相关性血小板减少症专家诊疗共识(2019版)[J]. 中国医学前沿杂志(电子版), 2020, 12(1): 51-58.
[7] 吴全睿, 赵永强, 储大同, 等. 重组人血小板生成素治疗肿瘤患者化疗后血小板减少症的疗效和安全性: Ⅱ/Ⅲ期及补充多中心随机对照临床试验的汇总分析[J]. 中国肿瘤生物治疗杂志, 2013, 20(6): 9.
[8] Xie CY, Zhao HJ, Bao XB, et al. Pharmacological characterization of hetrombopag, a novel orally active human thrombopoietin receptor agonist[J]. J Cell Mol Med, 2018, 22(11): 5367-5377. doi: 10.1111/jcmm.13809
[9] Thrombosis and Hemostasis Group of Chinese Society of Hematology, Chinese Medical Association. Chinese guideline on the diag-nosis and management of adult primary immune thrombocytopenia(version 2020)[J]. Chin J Hematol, 2020, 41(8): 617-623.
[10] Liu XG, Hou Y, Hou M. How we treat primary immune thrombocytopenia in adults[J]. J Hematol Oncol, 2023, 16(1): 4.
[11] Syed YY. Hetrombopag: first approval[J]. Drugs, 2021, 81(13): 1581-1585.
[12] Al-Samkari H. Thrombopoietin receptor agonists for chemotherapy-induced thrombocytopenia: a new solution for an old problem[J]. Hematology Am Soc Hematol Educ Program, 2022, 2022(1): 286-295.
[13] Al-Samkari H, Kolb-Sielecki J, Safina SZ, et al. Avatrombopag for chemotherapy-induced thrombocytopenia in patients with non-haematological malignancies: an international, randomised, double-blind, placebo-controlled, phase 3 trial[J]. Lancet Haematol, 2022, 9(3): e179-e189.
[14] 郭惠敏, 陈文娟. 艾曲波帕联合重组人血小板生成素治疗妇科肿瘤放化疗后血小板减少症的疗效[J]. 福建医药杂志, 2020, 42(6): 31-34.
[15] Mei H, Liu XF, Li Y, et al. A multicenter, randomized phase Ⅲ trial of hetrombopag: a novel thrombopoietin receptor agonist for the treatment of immune thrombocytopenia[J]. J Hematol Oncol, 2021, 14(1): 37.
[16] Mei H, Chen XQ, Zhou JF, et al. Safety and efficacy of hetrombopag in patients with chronic immune thrombocytopenia: a single-arm, open-label, multi-center phase 1 study[J]. Ann Transl Med, 2022, 10(2): 30.
[17] Li T, Liu QQ, Pu T, et al. Efficacy and safety of thrombopoietin receptor agonists in children and adults with persistent and chronic immune thrombocytopenia: a meta-analysis[J]. Expert Opin Pharmacother, 2023, 24(6): 763-774.
[18] Shen N, Qiao JB, Jiang YZ, et al. Safety of non-peptide thrombopoietin receptor agonists in patients with immune thrombocytopenia: a systematic review and meta-analysis of short-term double-blind randomized clinical trials[J]. Exp Ther Med, 2023, 26(2): 393.
[19] Dong Y, Xia ZN, Zhou J, et al. Risk of thrombotic events in immune thrombocytopenia patients treated with thrombopoietic agents: a systematic review and meta-analysis[J]. Thromb J, 2023, 21(1): 69.
-

计量
- 文章访问数: 212
- 施引文献: 0



 下载:
下载: