-
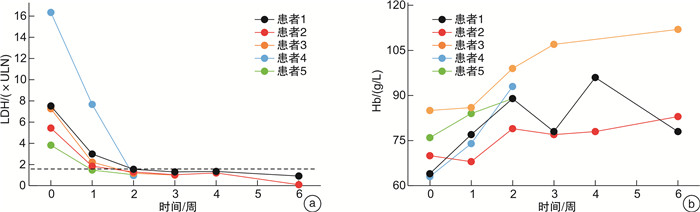
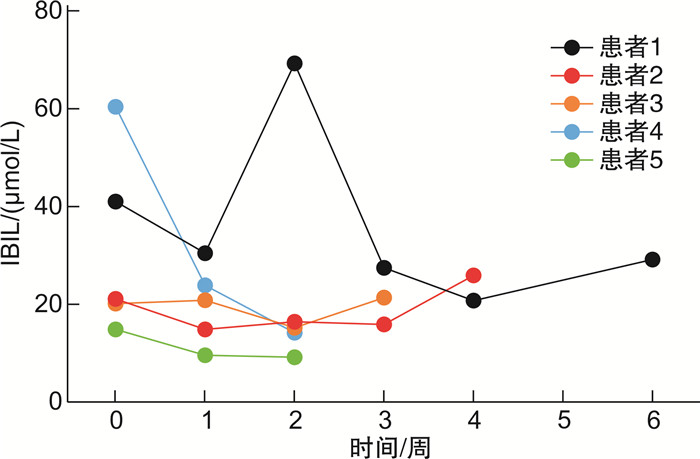
摘要: 目的 末端补体C5抑制剂依库珠单抗(eculizumab)被推荐为阵发性睡眠性血红蛋白尿症(paroxysmal nocturnal hemoglobinuria,PNH)的一线治疗方案。本研究探索了21例PNH患者接受依库珠单抗治疗的疗效及安全性。方法 使用依库珠单抗治疗21例溶血发作的PNH患者,评价患者溶血、贫血、肾功能及D-二聚体水平。结果 治疗后随访中位时间7天时,患者溶血、贫血及肾功能指标改善,D-二聚体水平下降,仅1例患者需要输血。长期规律应用依库珠单抗的PNH患者,在第2剂使用后4例(4/5,80%)达到溶血控制(乳酸脱氢酶 < 1.5×正常值上限),第3剂使用后全部达到溶血完全控制。9例仅接受1次依库珠单抗治疗,在随访28天时3例出现突破性溶血。12例提前接受脑膜炎球菌疫苗接种,另外9例紧急治疗者接受抗生素预防感染,随访中均未发生感染。结论 依库珠单抗能够有效控制PNH患者的溶血危机,改善肾功能,降低D-二聚体水平。紧急治疗未接受脑膜炎球菌疫苗接种者经预防性使用抗生素,可以避免感染。
-
关键词:
- 依库珠单抗 /
- 阵发性睡眠性血红蛋白尿症 /
- 溶血 /
- 感染 /
- 突破性溶血
Abstract: Objective Eculizumab as a terminal complement inhibitor is recommended as the first-line treatment for paroxysmal nocturnal hemoglobinuria(PNH). This study aimed to explore the efficacy and safety of eculizumab.Methods The indicators of hemolysis, anemia, kidney function, and the D-dimer in 21 PNH patients receiving with eculizumab treatment were retrospectively evaluated.Results After being treated with eculizumab, the hemolysis, anemia, and renal function of patients were improved and D-dimer levels declined compared to the baseline. Only one patient needed a blood transfusion. Five patients who were administered long-term regular eculizumab had achieved hemolysis control(lactate dehydrogenase < 1.5 upper limit of normal) after the third dose, and lactate dehydrogenase returned to normal levels. Three of nine PNH patients who only received eculizumab once had breakthrough hemolysis after 28 days. Among 21 patients, 12 cases received meningococcal vaccination in advance, 9 cases were treated urgently with antibiotics against infection, and all remained uninfected during the follow-up period.Conclusion The hemolytic breakthrough in PNH patients benefited from eculizumab in improving anemia, kidney function, and D-dimer levels. In emergency treatment, antibiotics are necessary to be given and could avoid infection.-
Key words:
- eculizumab /
- paroxysmal nocturnal hemoglobinuria /
- hemolysis /
- infection /
- breakthrough hemolysis
-

-
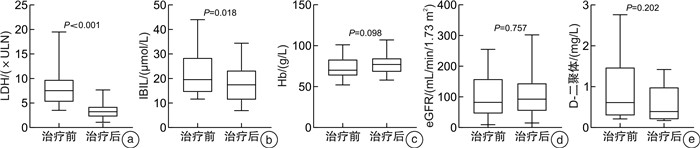
表 1 9例依库珠单抗治疗1次前后指标改变
指标 治疗前 治疗中位时间7天时 治疗中位时间28天时 P0 P1 P2 LDH/(×ULN) 9.52±4.62 4.31±1.77 7.01±9.05 0.006 0.173 0.953 Hb/(g/L) 79.89±15.81 82.44±15.02 92.22±19.70 0.628 0.091 0.114 eGFR/(mL/min/1.73 m2) 100.19±80.08 113.50±90.19 102.31±76.07 0.088 0.839 0.357 D-二聚体/(mg/L) 1.16±1.04 0.42±0.31 0.82±0.69 0.027 0.463 0.173 IBIL/(μmol/L) 22.34±7.97 18.53±6.24 28.82±28.01 0.436 0.893 0.686 P0、P1、P2分别为治疗中位时间7天时与治疗前、治疗中位时间28天时与治疗前、治疗中位时间28天时与治疗中位时间7天时实验室指标比较的P值。 -
[1] Risitano AM, Peffault de Latour R. How we('ll)treat paroxysmal nocturnal haemoglobinuria: diving into the future[J]. Br J Haematol, 2022, 196(2): 288-303. doi: 10.1111/bjh.17753
[2] Cancado RD, Araujo ADS, Sandes AF, et al. Consensus statement for diagnosis and treatment of paroxysmal nocturnal haemoglobinuria[J]. Hematol Transfus Cell Ther, 2021, 43(3): 341-348. doi: 10.1016/j.htct.2020.06.006
[3] 何广胜. 免疫抑制治疗再生障碍性贫血的选择[J]. 临床血液学杂志, 2016, 29(6): 874-876. https://lcxy.whuhzzs.com/article/doi/10.13201/j.issn.1004-2806.2016.11.005
[4] Szer J. C5 inhibition in PNH: still effective and safe[J]. Blood, 2024, 143(12): 1064-1065. doi: 10.1182/blood.2023023626
[5] Schrezenmeier H, Roth A, Araten DJ, et al. Baseline clinical characteristics and disease burden in patients with paroxysmal nocturnal hemoglobinuria(PNH): updated analysis from the International PNH Registry[J]. Ann Hematol, 2020, 99(7): 1505-1514. doi: 10.1007/s00277-020-04052-z
[6] Jang JH, Kim JS, Lim CTK, et al. Impact of Lactate Dehydrogenase and Hemoglobin Levels on Clinical Outcomes in Patients With Paroxysmal Nocturnal Hemoglobinuria: Results From the National Korean PNH Registry[J]. J Korean Medical Sci, 2024, 39(8): e81. doi: 10.3346/jkms.2024.39.e81
[7] Gurnari C, Awada H, Pagliuca S, et al. Paroxysmal nocturnal hemoglobinuria-related thrombosis in the era of novel therapies: a 2043-patient-year analysis[J]. Blood, 2024, 144(2): 145-155. doi: 10.1182/blood.2024023988
[8] Terriou L, Lee JW, Forsyth C, et al. Long-term effectiveness of eculizumab: Data from the International PNH Registry[J]. Eur J Haematol, 2023, 111(5): 796-804. doi: 10.1111/ejh.14080
[9] Gembillo G, Siligato R, Cernaro V, et al. Complement Inhibition Therapy and Dialytic Strategies in Paroxysmal Nocturnal Hemoglobinuria: The Nephrologist's Opinion[J]. J Clin Med, 2020, 9(5): 1261. doi: 10.3390/jcm9051261
[10] Macedo êS, Parente Filho SLA, Pro JDZ, et al. Renal involvement in paroxysmal nocturnal haemoglobinuria: a brief review of the literature[J]. Rev Assoc Med Bras(1992), 2018, 64(12): 1139-1146. doi: 10.1590/1806-9282.64.12.1139
[11] Ebert N, Bevc S, Bökenkamp A, et al. Assessment of kidney function: clinical indications for measured GFR[J]. Clin Kidney J, 2021, 14(8): 1861-1870. doi: 10.1093/ckj/sfab042
[12] Socie G, Caby-Tosi M, Marantz JL, et al. Eculizumab in paroxysmal nocturnal haemoglobinuria and atypical haemolytic uraemic syndrome: 10-year pharmacovigilance analysis[J]. Br J Haematol, 2019, 185(2): 297-310. doi: 10.1111/bjh.15790
[13] Brodsky RA. How I treat paroxysmal nocturnal hemoglobinuria[J]. Blood, 2021, 137(10): 1304-1309. doi: 10.1182/blood.2019003812
[14] Fishman J, Kuranz S, Yeh MM, et al. Changes in Hematologic Lab Measures Observed in Patients with Paroxysmal Nocturnal Hemoglobinuria Treated with C5 Inhibitors, Ravulizumab and Eculizumab: Real-World Evidence from a US Based EMR Network[J]. Hematol Rep, 2023, 15(2): 266-282. doi: 10.3390/hematolrep15020027
[15] Versmold K, Alashkar F, Raiser C, et al. Long-term outcomes of patients with paroxysmal nocturnal hemoglobinuria treated with eculizumab in a real-world setting[J]. Eur J Haematol, 2023, 111(1): 84-95. doi: 10.1111/ejh.13970
[16] Notaro R, Luzzatto L. Breakthrough Hemolysis in PNH with Proximal or Terminal Complement Inhibition[J]. N Engl J Med, 2022, 387(2): 160-166. doi: 10.1056/NEJMra2201664
[17] Shammo J, Gajra A, Patel Y, et al. Low Rate of Clinically Evident Extravascular Hemolysis in Patients with Paroxysmal Nocturnal Hemoglobinuria Treated with a Complement C5 Inhibitor: Results from a Large, Multicenter, US Real-World Study[J]. Blood Med, 2022, 13: 425-437. doi: 10.2147/JBM.S361863
[18] Oliver M, Patriquin C. Paroxysmal Nocturnal Hemoglobinuria: Current Management, Unmet Needs, and Recommendations[J]. J Blood Med, 2023, 14: 613-628. doi: 10.2147/JBM.S431493
-

计量
- 文章访问数: 193
- 施引文献: 0



 下载:
下载: