Clinical efficacy analysis of polymyxin B atomization treatment in severe blood disease patients with multidrug-resistant gram-negative pneumonia
-
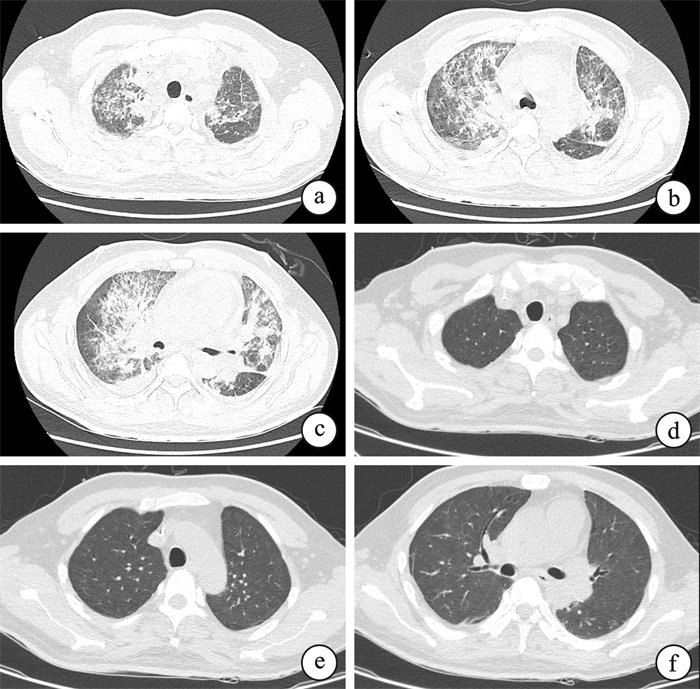
摘要: 目的 回顾性分析多重耐药(MDR)革兰阴性菌(GNB)感染重症肺炎的血液病患者加用多黏菌素B雾化治疗患者的临床资料。方法 收集华中科技大学同济医学院附属协和医院血液重症病房(HCU)2022年12月—2023年7月收治的48例MDR-GNB感染的重症肺炎的血液病患者资料。依据多黏菌素优化应用国际共识指南雾化吸入治疗(25 mg,12 h 1次,振动筛孔雾化吸入),观察患者使用疗程结束后的有效率、治疗前后感染指标C反应蛋白(CRP)及血清降钙素原(PCT)水平的变化。结果 48例患者痰/血培养或病原微生物二代测序结果显示:耐碳青霉烯阴沟肠杆菌/大肠埃希菌13例,鲍曼不动杆菌8例,铜绿假单胞菌6例,肺炎克雷伯菌4例,嗜麦芽窄食单胞菌4例,其他为少见病原菌或者疑似MDR感染患者;其中合并新型冠状病毒感染患者10例。动态分析治疗过程中感染指标CRP和血清PCT水平的变化:治疗后3 d CRP水平较治疗前开始下降[(94.06±71.30) mg/L vs (70.55±58.04)mg/L],治疗后7 d[(94.06±71.30)mg/L vs (46.40±40.93)mg/L,P < 0.01]以及治疗后14 d[(94.06±71.30)mg/L vs (47.93±51.08)mg/L,P < 0.01]显著下降,差异有统计学意义。PCT水平在治疗后7 d也开始出现明显下降[(5.15±12.14)μg/L vs (3.76±5.00)μg/L],与CRP变化一致。治疗前后,患者凝血功能、胆红素以及肌酐水平无明显变化,无肝肾功能损害(P>0.05)。结论 血液重症患者常合并重度免疫缺陷,治疗MDR-GNB感染的肺炎使用多黏菌素B联合其他抗生素治疗有效,感染指标在治疗14 d内呈动态下降趋势,可以提高挽救性治疗合并新型冠状病毒感染重症患者的疗效。
-
关键词:
- 多黏菌素B /
- 雾化吸入 /
- 多重耐药革兰阴性菌肺炎 /
- 血液重症患者 /
- 临床疗效
Abstract: Objective This study was conducted to retrospectively analyze the clinical data of blood disease patients with severe pneumonia caused by multidrug-resistant(MDR) gram negative bacteria(GNB) who were treated with nebulization of polymyxin B.Methods Collect data of 48 blood disease patients with severe pneumonia caused by MDR-GNB infection who were admitted to the Hematology Intensive Care Unit(HCU) of Wuhan Union Hospital from December 2022 to July 2023. According to the international consensus guidelines for optimizing the application of polymyxin, nebulization therapy(25 mg, once every 12 hours, vibrating sieve nebulization inhalation) was applied to these patients. The effective rate, changes in infection markers such as C-reactive protein(CRP) and serum procalcitonin(PCT) levels were observed after the treatment course.Results The results of Sputum/blood cultures or second-generation sequencing of pathogenic microorganisms from 48 patients showed that 13 patients were infected by carbapenem resistant Enterobacter cloacae/Escherichia coli, 8 patients were infected with Acinetobacter baumannii, 6 patients were infected with Pseudomonas aeruginosa, 4 patients were infected with Klebsiella pneumoniae, 4 patients were infected with Stenotrophomonas maltophilia, and others were infected with rare pathogenic bacteria or suspected MDR-GNB; Among them, 10 patients had co-infection with COVID-19. Dynamic analysis of changes in infection markers CRP and PCT during treatment indicated that CRP levels began to decrease after 3 days of treatment[(94.06±71.30)mg/L vs (70.55±58.04) mg/L], and kept decreasing at 7 days [(94.06±71.30) mg/L vs (46.40±40.93) mg/L, P < 0.01]and 14 days[(94.06±71.30) mg/L vs (47.93±51.08) mg/L, P < 0.01]. The results above is statistically significant. PCT levels also began to decrease obviously after 7 days of treatment[(5.15±12.14) μg/L vs (3.76±5.00) μg/L], which was consistent with changes in CRP. During the entire course of treatment, there were no significant changes in coagulation function, bilirubin, and creatinine levels among these patients, and no liver or kidney function damage(P>0.05).Conclusion Patients with severe blood disease are usually complicated with severe immune deficiency. Polymyxin B combined with other antibiotics is effective in the treatment of MDR-GNB infected pneumonia, as infection markers decreased dynamically within 14 days treatment of polymyxin B atomization. Besides, polymyxin B atomization treatment can improve the efficacy of rescue treatment for patients with severe COVID-19 infection. -

-
表 1 MDR-GNB肺炎诊断标准
条目 备注 ①胸部X线或CT显示新出现或进展性的浸润影、实变影或磨玻璃影 ②痰/血培养或病原微生物二代测序检出MDR-GNB ①②为必须满足,③④⑤需满足2条或2条以上 ③发热,体温>38℃ ④有脓性气道分泌物 ⑤外周血白细胞计数>10×109/L或 < 4×109/L 表 2 患者基本资料
例(%),X±S 基本资料 数据 性别 男 27(56.25) 女 21(43.75) 年龄/岁 46.96±18.25 原发疾病 急性白血病 34(70.83) 淋巴瘤 4(8.33) 再生障碍性贫血 3(6.25) 多发性骨髓瘤 2(4.17) 骨髓增生异常综合征 2(4.17) 慢性粒细胞白血病 1(2.08) 噬血细胞综合征 1(2.08) 血栓性血小板减少性紫癜 1(2.08) 感染病原体 耐碳青霉烯阴沟肠杆菌/大肠埃希菌 13(27.08) 鲍曼不动杆菌 8(16.67) 铜绿假单胞菌 6(12.50) 肺炎克雷伯菌 4(8.33) 嗜麦芽窄食单胞菌 4(8.33) 少见病原菌或者疑似MDR感染 13(27.08) 住院时间/d 13±8 表 3 雾化治疗期间患者炎症和脏器功能指标动态变化
X±S 指标 治疗后时间/d 0 3 7 14 血常规 白细胞计数/(×109/L) 10.63±19.59 8.03±8.40 10.44±11.32 6.90±8.85 中性粒细胞/(×109/L) 5.83±7.39 5.65±6.39 7.76±8.98 5.51±7.20 凝血功能 凝血酶原时间/s 14.76±3.12 14.83±2.26 13.73±3.47 14.42±4.83 纤维蛋白原/(g/L) 3.51±1.66 3.28±1.76 3.35±1.85 3.03±1.26 肝功能 总胆红素/(μmol/L) 18.62±14.66 / 19.89±32.10 / 谷丙转氨酶/(U/L) 31.30±47.69 / 30.79±25.61 / 肾功能 血清肌酐/(μmol/L) 80.40±124.40 / 70.72±53.66 / 肾小球滤过率/(mL/min/1.73 m2) 117.80±70.98 / 106.70±37.93 / 感染指标 CRP/(mg/L) 94.06±71.30 70.55±58.04 46.40±40.931) 47.93±51.081) PCT/(μg/L) 5.15±12.14 4.42±7.21 3.76±5.00 1.96±2.29 注:与治疗后第0天比较,1)P < 0.05。 表 4 不同分组患者治疗有效率
变量 例数 治疗有效例数 有效率/% 性别 男 27 15 55.56 女 21 13 61.90 年龄/岁 < 65 39 26 66.67 ≥65 9 2 22.22 原发疾病 急性白血病 34 19 55.88 淋巴瘤 4 2 50.00 再生障碍性贫血 3 2 66.67 多发性骨髓瘤 2 2 100.00 骨髓增生异常综合征 2 0 0 慢性粒细胞白血病 1 1 100.00 噬血细胞综合征 1 1 100.00 血栓性血小板减少性紫癜 1 1 100.00 感染病原体 耐碳青霉烯阴沟肠杆菌/大肠埃希菌 13 8 61.54 鲍曼不动杆菌 8 5 62.50 铜绿假单胞菌 6 3 50.00 肺炎克雷伯菌 4 2 50.00 嗜麦芽窄食单胞菌 4 1 25.00 少见病原菌或者疑似MDR感染 13 8 61.54 治疗前中性粒细胞计数 < 0.5×109/L 8 3 37.50 ≥0.5×109/L 40 25 62.50 治疗前CRP < 100 mg/L 28 16 57.14 ≥100 mg/L 20 12 60.00 治疗前PCT < 2 μg/L 30 20 66.67 ≥2 μg/L 18 8 44.44 -
[1] El-Sayed Ahmed MAE, Zhong LL, Shen C, et al. Colistin and its role in the era of antibiotic resistance: an extended review(2000-2019)[J]. Emerg Microbes Infect, 2020, 9(1): 868-885. doi: 10.1080/22221751.2020.1754133
[2] Boisson M, Jacobs M, Grégoire N, et al. Comparison of intrapulmonary and systemic pharmacokinetics of colistin methanesulfonate(CMS)and colistin after aerosol delivery and intravenous administration of CMS in critically ill patients[J]. Antimicrob Agents Chemother, 2014, 58(12): 7331-7339. doi: 10.1128/AAC.03510-14
[3] Boisson M, Grégoire N, Cormier M, et al. Pharmacokinetics of nebulized colistin methanesulfonate in critically ill patients[J]. J Antimicrob Chemother, 2017, 72(9): 2607-2612. doi: 10.1093/jac/dkx167
[4] Giamarellou H, Poulakou G. Multidrug-resistant Gram-negative infections: what are the treatment options? [J]. Drugs, 2009, 69(14): 1879-1901. doi: 10.2165/11315690-000000000-00000
[5] Rigatto MH, Oliveira MS, Perdigão-Neto LV, et al. Multicenter Prospective Cohort Study of Renal Failure in Patients Treated with Colistin versus Polymyxin B[J]. Antimicrob Agents Chemother, 2016, 60(4): 2443-2449. doi: 10.1128/AAC.02634-15
[6] Ding P, Li H, Nan Y, et al. Outcome of intravenous and inhaled polymyxin B treatment in patients with multidrug-resistant gram-negative bacterial pneumonia[J]. Int J Antimicrob Agents, 2024, 64(4): 107293. doi: 10.1016/j.ijantimicag.2024.107293
[7] Wu Z, Zhang S, Cao Y, et al. Comparison of the clinical efficacy and toxicity of nebulized polymyxin monotherapy and combined intravenous and nebulized polymyxin for the treatment of ventilator-associated pneumonia caused by carbapenem-resistant gram-negative bacteria: a retrospective cohort study[J]. Front Pharmacol, 2023, 14: 1209063. doi: 10.3389/fphar.2023.1209063
[8] 周丽丽, 李彩婷, 翁钦永, 等. 静脉滴注联合雾化吸入多黏菌素B治疗多重耐药革兰阴性菌肺炎的临床分析[J]. 中华危重病急救医学, 2021, 33(4): 416-420. doi: 10.3760/cma.j.cn121430-20201215-00753
[9] 中国医药教育协会感染疾病专业委员会, 中华医学会呼吸病学分会, 中华医学会重症医学分会, 等. 中国多黏菌素类抗菌药物临床合理应用多学科专家共识[J]. 中华结核和呼吸杂志, 2021, 44(4): 292-310. doi: 10.3760/cma.j.cn112147-20201109-01091
[10] Trecarichi EM, Giuliano G, Cattaneo C, et al. Bloodstream infections due to gram-negative bacteria in patients with hematologic malignancies: updated epidemiology and risk factors for multidrug-resistant strains in an Italian perspective survey[J]. Int J Antimicrob Agents, 2023, 61(6): 106806. doi: 10.1016/j.ijantimicag.2023.106806
[11] Wang J, Mu M, Zhu J, et al. Adult acute leukemia patients with gram-negative bacteria bloodstream infection: risk factors and outcomes of antibiotic-resistant bacteria[J]. Ann Hematol, 2024, 103(10): 4021-4031. doi: 10.1007/s00277-024-05866-x
[12] Wang S, Song Y, Shi N, et al. Characteristics, Outcomes, and Clinical Indicators of Bloodstream Infections in Neutropenic Patients with Hematological Malignancies: A 7-Year Retrospective Study[J]. Infect Drug Resist, 2023, 16: 4471-4487. doi: 10.2147/IDR.S413454
[13] Zhou Y, Wang G, Zhao Y, et al. Efficacy and safety of different polymyxin-containing regimens for the treatment of pneumonia caused by multidrug-resistant gram-negative bacteria: a systematic review and network meta-analysis[J]. Crit Care, 2024, 28(1): 239. doi: 10.1186/s13054-024-05031-w
[14] 中华医学会血液学分会感染学组, 中华医学会血液学分会淋巴细胞疾病学组, 中国临床肿瘤学会(CSCO)抗淋巴瘤联盟. 血液肿瘤免疫及靶向药物治疗相关性感染预防及诊治中国专家共识(2021年版)[J]. 中华血液学杂志, 2021, 42(9): 717-727.
[15] Baden LR, Swaminathan S, Almyroudis NG, et al. Prevention and Treatment of Cancer-Related Infections, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology[J]. J Natl Compr Canc Netw, 2024, 22(9): 617-644. doi: 10.6004/jnccn.2024.0056
[16] Zhang X, Qi S, Duan X, et al. Clinical outcomes and safety of polymyxin B in the treatment of carbapenem-resistant Gram-negative bacterial infections: a real-world multicenter study[J]. J Transl Med, 2021, 19(1): 431. doi: 10.1186/s12967-021-03111-x
[17] Wu X, Zhu Y, Chen Q, et al. Tigecycline Therapy for Nosocomial Pneumonia due to Carbapenem-Resistant Gram-Negative Bacteria in Critically Ill Patients Who Received Inappropriate Initial Antibiotic Treatment: A Retrospective Case Study[J]. Biomed Res Int, 2016, 2016: 8395268.
[18] Geng TT, Xu X, Huang M. High-dose tigecycline for the treatment of nosocomial carbapenem-resistant Klebsiella pneumoniae bloodstream infections: A retrospective cohort study[J]. Medicine, 2018, 97(8): e9961. doi: 10.1097/MD.0000000000009961
[19] Alraddadi BM, Saeedi M, Qutub M, et al. Efficacy of ceftazidime-avibactam in the treatment of infections due to Carbapenem-resistant Enterobacteriaceae[J]. BMC Infect Dis, 2019, 19(1): 772. doi: 10.1186/s12879-019-4409-1
[20] Cai Y, Lee W, Kwa AL. Polymyxin B versus colistin: an update[J]. Expert Rev Anti Infect Ther, 2015, 13(12): 1481-1497. doi: 10.1586/14787210.2015.1093933
[21] Kassamali Z, Danziger L. To B or not to B, that is the question: is it time to replace colistin with polymyxin B?[J]. Pharmacotherapy, 2015, 35(1): 17-21. doi: 10.1002/phar.1510
[22] Qu J, Qi TT, Qu Q, et al. Polymyxin B-Based Regimens for Patients Infected with Carbapenem-Resistant Gram-Negative Bacteria: Clinical and Microbiological Efficacy, Mortality, and Safety[J]. Infect Drug Resist, 2022, 15: 1205-1218. doi: 10.2147/IDR.S357746
[23] Falagas ME, Kyriakidou M, Voulgaris GL, et al. Clinical use of intravenous polymyxin B for the treatment of patients with multidrug-resistant Gram-negative bacterial infections: An evaluation of the current evidence[J]. J Glob Antimicrob Resist, 2021, 24: 342-359. doi: 10.1016/j.jgar.2020.12.026
[24] Azad MAK, Nation RL, Velkov T, et al. Mechanisms of Polymyxin-Induced Nephrotoxicity[J]. Adv Exp Med Biol, 2019, 1145: 305-319.
[25] Lee DH, Kim SY, Kim YK, et al. Intrapulmonary and Systemic Pharmacokinetics of Colistin Following Nebulization of Low-Dose Colistimethate Sodium in Patients with Ventilator-Associated Pneumonia Caused by Carbapenem-Resistant Acinetobacter baumannii[J]. Antibiotics(Basel), 2024, 13(3): 258.
[26] Shi R, Fu Y, Gan Y, et al. Use of polymyxin B with different administration methods in the critically ill patients with ventilation associated pneumonia: a single-center experience[J]. Front Pharmacol, 2023, 14: 1222044. doi: 10.3389/fphar.2023.1222044
[27] Ahmed MU, Azad MAK, Li M, et al. Polymyxin-Induced Metabolic Perturbations in Human Lung Epithelial Cells[J]. Antimicrob Agents Chemother, 2021, 65(9): e0083521. doi: 10.1128/AAC.00835-21
[28] Ahmed MU, Velkov T, Lin YW, et al. Potential Toxicity of Polymyxins in Human Lung Epithelial Cells[J]. Antimicrob Agents Chemother, 2017, 61(6): e02690-16.
[29] Zhu L, Wang L, Zhang Y, et al. Fatal hemorrhagic pneumonia in patients with hematologic diseases and Stenotrophomonas maltophilia bacteremia: a retrospective study[J]. BMC Infect Dis, 2021, 21(1): 723. doi: 10.1186/s12879-021-06420-0
[30] Turkoglu M, Mirza E, Tunçcan ÖG, et al. Acinetobacter baumannii infection in patients with hematologic malignancies in intensive care unit: risk factors and impact on mortality[J]. J Crit Care, 2011, 26(5): 460-467. doi: 10.1016/j.jcrc.2011.04.007
[31] Kim SH, Cha MK, Kang CI, et al. Pathogenic significance of hemorrhagic pneumonia in hematologic malignancy patients with Stenotrophomonas maltophilia bacteremia: clinical and microbiological analysis[J]. Eur J Clin Microbiol Infect Dis, 2019, 38(2): 285-295. doi: 10.1007/s10096-018-3425-1
[32] Huang C, Kuo S, Lin L. Hemorrhagic Pneumonia Caused by Stenotrophomonas maltophilia in Patients with Hematologic Malignancies-A Systematic Review and Meta-Analysis[J]. Medicina(Kaunas), 2024, 60(1): 162.
-




 下载:
下载: