Analysis of the efficacy of high-dose ulinastatin in the treatment of neutropenic sepsis patients
-
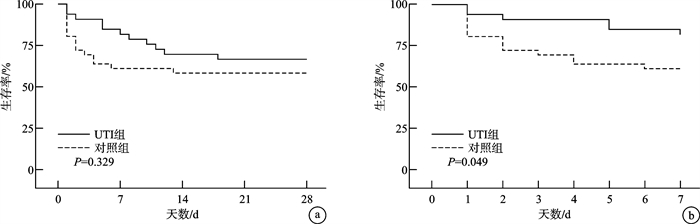
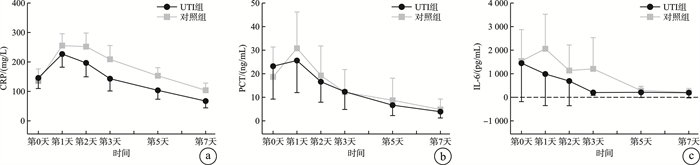
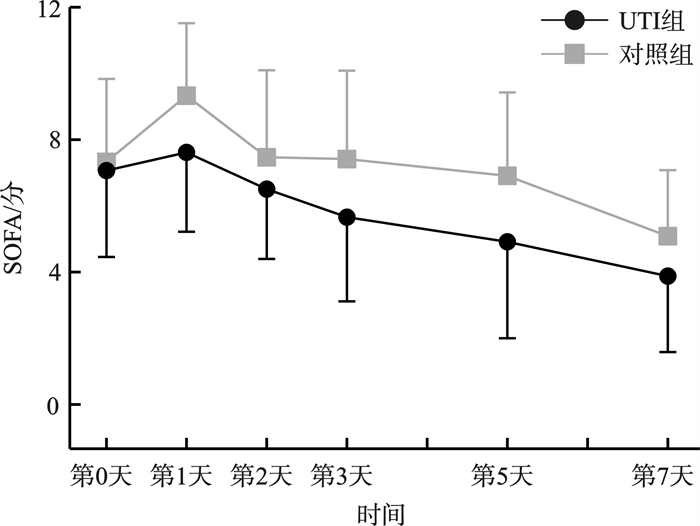
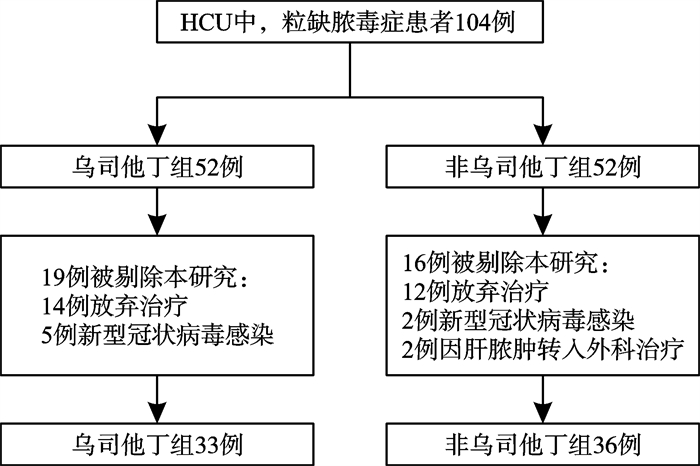
摘要: 目的 观察高剂量乌司他丁对中性粒细胞缺乏伴脓毒症(粒缺脓毒症)患者炎症指标、脏器功能以及预后的影响。方法 选取2020年1月—2024年11月哈尔滨医科大学附属第一医院血液肿瘤重症监护病房(hematological intensive care unit,HCU)收治的104例粒缺脓毒症患者,以随机数字表法分为乌司他丁组(UTI组)和非乌司他丁组(对照组),经筛选最终入选UTI组33例,对照组36例。UTI组在常规治疗基础上应用乌司他丁40万U,2次/d,治疗7 d;对照组仅给予常规治疗。比较两组间炎症指标白细胞介素6(IL-6)、C反应蛋白(CRP)和降钙素原(PCT)水平变化,序贯器官衰竭评分(SOFA评分)变化以及预后情况。结果 治疗后第1天,仅UTI组IL-6呈下降趋势,而对照组IL-6、两组PCT和CRP以及SOFA评分均升至峰值;治疗后第2~7天,两组上述指标均呈持续降低趋势。与对照组比较,UTI组CRP水平及SOFA评分下降更明显(P=0.042,P=0.034);两组间PCT和IL-6水平差异无统计学意义。两组28 d死亡率差异无统计学意义,而与对照组比较,UTI组的7 d死亡率较低(P=0.049)。结论 高剂量乌司他丁治疗粒缺脓毒症疗效较好,可改善粒缺脓毒症早期高炎症反应导致的脏器损伤,从而延缓疾病进展,降低粒缺脓毒症患者的7 d死亡率,但未降低28 d死亡率。Abstract: Objective This study was conducted to analyze the efficacy of high-dose ulinastatin(UTI) on inflammatory indicators, organ function and prognosis of neutropenic sepsis patients.Methods A total of 104 neutropenic sepsis patients were selected from January 2020 to November 2024, in the Hematological Intensive Care Unit(HCU), the First Affiliated Hospital of Harbin Medical University. Patients were divided into UTI group and non-ulinastatin group(control group) by random number table method. After screening, the UTI group included 33 cases, and the control group included 36 cases. The patients of UTI group received 400 000 U of Ulastatin, twice a day for 7 days. And the control group only received conventional treatment. The changes of IL-6, CRP, PCT and SOFA score were compared between the two groups.Results On the first day after UTI treatment, only IL-6 in the UTI group showed a decreasing trend, while IL-6 in the control group, PCT, CRP and SOFA score in both groups rose to the peak; From the second day to the seventh day following UTI treatment, both groups displayed a consistent downward trend. The CRP level(P=0.042) and SOFA score(P=0.034) of UTI group dropped more sharply than those of the control group. There was no significant difference in PCT and IL-6 between the two groups. The UTI group had a lower 7-day mortality rate than the control group(P=0.049), however there was no significant difference in the 28-day mortality rate between the two groups.Conclusion In the early stage of neutropenic sepsis, high-dose ulinastatin could ameliorate the damage to organs caused by high inflammatory response, delay the progression of the disease, and lower the 7-day mortality rate of neutropenic sepsis, but not the 28-day mortality rate.
-
Key words:
- ulinastatin /
- neutropenia /
- sepsis
-

-
表 1 粒缺脓毒症患者一般资料
例(%),M(P25,P75),X±S 基本特征 UTI组(n=33) 对照组(n=36) P 性别 0.265 男 20(28.99) 17(24.64) 女 13(18.84) 19(27.54) 年龄/岁 62.00(44.00,68.50) 56.50(39.00,60.00) 0.146 原发疾病 0.953 白血病 16(23.19) 18(26.09) 骨髓增生异常综合征 7(10.14) 7(10.14) 再生障碍性贫血 4(5.80) 4(5.80) 淋巴瘤 3(4.35) 5(7.25) 非血液系统疾病 3(4.35) 2(2.90) 生命体征 体温/℃ 37.30(36.72,38.17) 38.05(37.05,39.07) 0.102 呼吸频率/(次/min) 26.42±5.64 28.19±8.54 0.145 心率/(次/min) 114.72±23.58 118.00±24.39 0.574 脓毒性休克 25(75.76) 28(77.78) 0.843 SOFA评分/分 7.58±2.63 8.08±2.66 0.429 实验室检查 WBC/(×109/L) 0.75(0.21,1.35) 0.33(0.11,0.88) 0.101 ANC/(×109/L) 0.12(0.02,0.75) 0.02(0,0.32) 0.020 HGB/(g/L) 71.00(60.50,82.00) 69.97(51.01,80.42) 0.259 PLT/(×109/L) 21.00(6.53,44.50) 13.00(5.50,27.59) 0.171 ALT/(U/L) 25.50(13.35,40.80) 33.80(12.20,66.70) 0.469 TBIL/(μmol/L) 13.80(9.83,25.85) 22.70(11.70,34.30) 0.111 Cr/(μmol/L) 110.00(76.50,184.00) 67.10(47.00,94.20) 0.004 PT/s 14.20(12.40,16.30) 15.10(13.40,17.80) 0.210 D二聚体/(mg/L) 4.56(2.60,8.60) 2.83(1.35,13.13) 0.422 PCT/(ng/mL) 7.00(0.86,35.50) 4.34(0.45,29.77) 0.407 CRP/(mg/L) 138.50(76.45,197.00) 139.00(96.40,204.00) 0.975 HCU住院时间/d 5(3,9) 3(2,6) 0.034 HCU中死亡人数 11(33.33) 15(41.67) 0.475 中位死亡时间/d 6(2,11) 2(1,4) 0.019 表 2 乌司他丁治疗期间患者炎症和脏器功能指标动态变化
X±S 指标 组别 例数 治疗时间 P 第0天 第1天 第2天 第3天 第5天 第7天 PCT/(ng/mL) UTI组 27 23.22±35.37 25.61±34.41 16.64±22.09 12.39±19.06 6.68±11.22 3.91±6.77 0.852 对照组 21 18.68±28.61 30.81±34.75 19.27±28.29 12.17±21.70 8.74±21.20 4.85±10.03 CRP/(mg/L) UTI组 27 145.86±90.91 226.84±112.92 196.78±118.99 143.13±105.34 103.84±76.59 67.36±58.53 0.042 对照组 21 135.96±91.32 255.50±91.03 252.08±104.17 209.12±105.69 153.19±61.68 103.93±55.21 IL-6/(pg/mL) UTI组 9 1 447.93±2 122.96 988.78±1747.49 690.12±1361.65 203.81±161.13 210.02±287.29 194.30±285.59 0.215 对照组 10 1 539.82±1 861.59 2 058.15±2 047.14 1 135.45±1 517.48 1 207.27±1 848.26 283.75±252.07 210.73±234.38 SOFA/分 UTI组 27 7.07±2.61 7.62±2.40 6.51±2.11 5.66±2.54 4.92±2.92 3.88±2.29 0.034 对照组 21 7.33±2.51 9.33±2.19 7.47±2.63 7.42±2.67 6.91±2.52 5.09±1.99 -
[1] Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock(Sepsis-3)[J]. JAMA, 2016, 315(8): 801-810. doi: 10.1001/jama.2016.0287
[2] Giamarellos-Bourboulis EJ, Aschenbrenner AC, Bauer M, et al. The pathophysiology of sepsis and precision-medicine-based immunotherapy[J]. Nat Immunol, 2024, 25(1): 19-28. doi: 10.1038/s41590-023-01660-5
[3] Reilly JP, Anderson BJ, Hudock KM, et al. Neutropenic sepsis is associated with distinct clinical and biological characteristics: a cohort study of severe sepsis[J]. Crit Care, 2016, 20(1): 222. doi: 10.1186/s13054-016-1398-y
[4] 中华医学会血液学分会, 中国医师协会血液科医师分会. 中国中性粒细胞缺乏伴发热患者抗菌药物临床应用指南(2020年版)[J]. 中华血液学杂志, 2020, 41(12): 969-978. doi: 10.3760/cma.j.issn.0253-2727.2020.12.001
[5] Kanayama S, Yamada Y, Onogi A, et al. Bikunin suppresses expression of pro-inflammatory cytokines induced by lipopolysaccharide in neutrophils[J]. J Endotoxin Res, 2007, 13(6): 369-376. doi: 10.1177/0968051907086464
[6] Liu S, Xu J, Gao Y, et al. Multi-organ protection of ulinastatin in traumatic cardiac arrest model[J]. World J Emerg Surg, 2018, 13: 51. doi: 10.1186/s13017-018-0212-3
[7] 刘兆辉, 吴锦涛, 张友来, 等. 高剂量乌司他丁在严重烧伤治疗中的研究进展[J]. 实用临床医学, 2022, 23(5): 130-134.
[8] Chen Q, Hu C, Liu Y, et al. Safety and tolerability of high-dose ulinastatin after 2-hour intravenous infusion in adult healthy Chinese volunteers: A randomized, double-blind, placebo-controlled, ascending-dose study[J]. PLoS One, 2017, 12(5): e0177425. doi: 10.1371/journal.pone.0177425
[9] Huang H, Hu PF, Sun LL, et al. Treatment of patients with Covid-19 with a high dose of ulinastatin[J]. Exp Ther Med, 2022, 23(2): 121.
[10] Kochanek M, Schalk E, von Bergwelt-Baildon M, et al. Management of sepsis in neutropenic cancer patients: 2018 guidelines from the Infectious Diseases Working Party(AGIHO)and Intensive Care Working Party(iCHOP)of the German Society of Hematology and Medical Oncology(DGHO)[J]. Ann Hematol, 2019, 98(5): 1051-1069. doi: 10.1007/s00277-019-03622-0
[11] 曹钰, 柴艳芬, 邓颖, 等. 中国脓毒症/脓毒性休克急诊治疗指南(2018)[J]. 感染、炎症、修复, 2019, 20(1): 3-22. doi: 10.3969/j.issn.1672-8521.2019.01.001
[12] Xie J, Wang H, Kang Y, et al. The Epidemiology of Sepsis in Chinese ICUs: A National Cross-Sectional Survey[J]. Crit Care Med, 2020, 48(3): e209-e218. doi: 10.1097/CCM.0000000000004155
[13] Na SJ, Oh DK, Park S, et al. Clinical Characteristics and Outcomes of Neutropenic Sepsis: A Multicenter Cohort Study[J]. Shock, 2022, 57(5): 659-665. doi: 10.1097/SHK.0000000000001907
[14] 中国研究型医院学会休克与脓毒症专业委员会, 中国人民解放军重症医学专业委员会, 重症免疫研究协作组, 等. 脓毒症免疫抑制诊治专家共识[J]. 中华危重病急救医学, 2020, 32(11): 1281-1289. doi: 10.3760/cma.j.cn121430-20201123-00719
[15] Xu Q, Yan Q, Chen S. Use of ulinastatin was associated with reduced mortality in critically ill patients with sepsis[J]. J Thorac Dis, 2019, 11(5): 1911-1918. doi: 10.21037/jtd.2019.05.03
-




 下载:
下载: