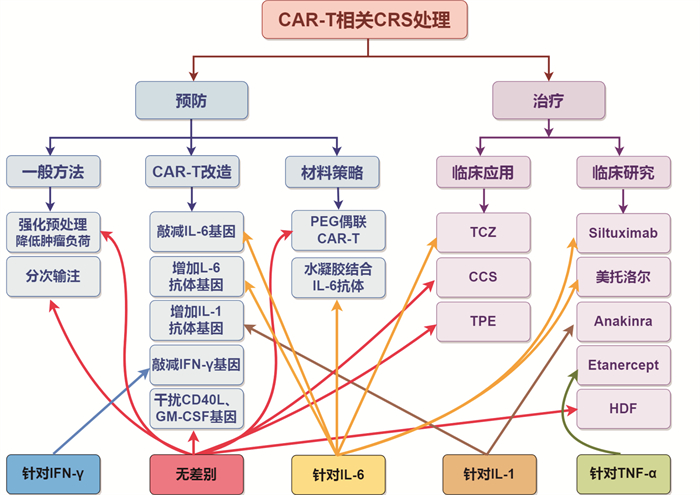
-
摘要: 细胞因子释放综合征(cytokine release syndrome,CRS)是嵌合抗原受体(chimeric antigen receptor,CAR)T细胞免疫治疗最主要的并发症,其发病涉及巨噬细胞等释放大量细胞因子损伤相应器官。为尽早识别和应对CRS,探索其预测指标的研究正在积极进行中,新的预防和治疗方法也不断涌现。Abstract: Cytokine release syndrome (CRS) represents a common and critical complication associated with chimeric antigen receptor (CAR) T cell immunotherapy, characterized by the pathogenesis involving macrophage-mediated cytokine overproduction, which subsequently leads to organ damage.To facilitate early identification and intervention of CRS, ongoing research is focused on elucidating its predictive markers, alongside the development of novel preventive and therapeutic strategies.
-

-
[1] Hao Z, Li R, Meng L, et al. Macrophage, the potential key mediator in CAR-T related CRS[J]. Exp Hematol Oncol, 2020, 9: 15. doi: 10.1186/s40164-020-00171-5
[2] Giavridis T, van der Stegen SJC, Eyquem J, et al. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade[J]. Nat Med, 2018, 24(6): 731-738. doi: 10.1038/s41591-018-0041-7
[3] Sterner RM, Sakemura R, Cox MJ, et al. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts[J]. Blood, 2019, 133(7): 697-709. doi: 10.1182/blood-2018-10-881722
[4] Sachdeva M, Duchateau P, Depil S, et al. Granulocyte-macrophage colony-stimulating factor inactivation in CAR T-cells prevents monocyte-dependent release of key cytokine release syndrome mediators[J]. J Biol Chem, 2019, 294(14): 5430-5437. doi: 10.1074/jbc.AC119.007558
[5] Ingelfinger F, De Feo D, Becher B. GM-CSF: Master regulator of the T cell-phagocyte interface during inflammation[J]. Semin Immunol, 2021, 54: 101518. doi: 10.1016/j.smim.2021.101518
[6] Dibas A, Rhiel M, Patel VB, et al. Cell-Based Models of 'Cytokine Release Syndrome' Endorse CD40 L and Granulocyte-Macrophage Colony-Stimulating Factor Knockout in Chimeric Antigen Receptor T Cells as Mitigation Strategy[J]. Cells, 2023, 12(21): 2581. doi: 10.3390/cells12212581
[7] Liu Y, Fang Y, Chen X, et al. Gasdermin E-mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome[J]. Sci Immunol, 2020, 5(43): eaax7969. doi: 10.1126/sciimmunol.aax7969
[8] Norelli M, Camisa B, Barbiera G, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells[J]. Nat Med, 2018, 24(6): 739-748. doi: 10.1038/s41591-018-0036-4
[9] Chen Y, Li R, Shang S, et al. Therapeutic Potential of TNFα and IL1β Blockade for CRS/ICANS in CAR-T Therapy via Ameliorating Endothelial Activation[J]. Front Immunol, 2021, 12: 623610. doi: 10.3389/fimmu.2021.623610
[10] Obstfeld AE, Frey NV, Mansfield K, et al. Cytokine release syndrome associated with chimeric-antigen receptor T-cell therapy: clinicopathological insights[J]. Blood, 2017, 130(23): 2569-2572. doi: 10.1182/blood-2017-08-802413
[11] Kang S, Tanaka T, Narazaki M, et al. Targeting Interleukin-6 Signaling in Clinic[J]. Immunity, 2019, 50(4): 1007-1023. doi: 10.1016/j.immuni.2019.03.026
[12] Maude SL, Barrett D, Teachey DT, et al. Managing cytokine release syndrome associated with novel T cell-engaging therapies[J]. Cancer J, 2014, 20(2): 119-122. doi: 10.1097/PPO.0000000000000035
[13] Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages[J]. Nat Rev Immunol, 2020, 20(6): 355-362. doi: 10.1038/s41577-020-0331-4
[14] Shao M, Yu Q, Teng X, et al. CRS-related coagulopathy in BCMA targeted CAR-T therapy: a retrospective analysis in a phase Ⅰ/Ⅱ clinical trial[J]. Bone Marrow Transplant, 2021, 56(7): 1642-1650. doi: 10.1038/s41409-021-01226-9
[15] Mantovani A, Dinarello CA, Molgora M, et al. Interleukin-1 and Related Cytokines in the Regulation of Inflammation and Immunity[J]. Immunity, 2019, 50(4): 778-795. doi: 10.1016/j.immuni.2019.03.012
[16] Karki R, Sharma BR, Tuladhar S, et al. Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes[J]. Cell, 2021, 184(1): 149-168. e17. doi: 10.1016/j.cell.2020.11.025
[17] Qi J, Lv X, Chen J, et al. TNF-α increases the risk of bleeding in patients after CAR T-cell therapy: A bleeding model based on a real-world study of Chinese CAR T Working Party[J]. Hematol Oncol, 2022, 40(1): 63-71.
[18] Kieler M, Hofmann M, Schabbauer G. More than just protein building blocks: how amino acids and related metabolic pathways fuel macrophage polarization[J]. FEBS J, 2021, 288(12): 3694-3714. doi: 10.1111/febs.15715
[19] Read JA, Rouce RH, Mo F, et al. Apoptosis of Hematopoietic Stem Cells Contributes to Bone Marrow Suppression Following Chimeric Antigen Receptor T Cell Therapy[J]. Transplant Cell Ther, 2023, 29(3): 165. e1-165. e7. doi: 10.1016/j.jtct.2022.12.020
[20] Wei Z, Cheng Q, Xu N, et al. Investigation of CRS-associated cytokines in CAR-T therapy with meta-GNN and pathway crosstalk[J]. BMC Bioinformatics, 2022, 23(1): 373. doi: 10.1186/s12859-022-04917-2
[21] Wang J, Mou N, Yang Z, et al. Efficacy and safety of humanized anti-CD19-CAR-T therapy following intensive lymphodepleting chemotherapy for refractory/relapsed B acute lymphoblastic leukaemia[J]. Br J Haematol, 2020, 191(2): 212-222. doi: 10.1111/bjh.16623
[22] Teachey DT, Lacey SF, Shaw PA, et al. Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-cell Therapy for Acute Lymphoblastic Leukemia[J]. Cancer Discov, 2016, 6(6): 664-679. doi: 10.1158/2159-8290.CD-16-0040
[23] Hay KA, Hanafi LA, Li D, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy[J]. Blood, 2017, 130(21): 2295-2306. doi: 10.1182/blood-2017-06-793141
[24] Goldsmith SR, Shouse G, Wong FL, et al. Clonal Hematopoiesis is Associated with Severe Cytokine Release Syndrome in Patients Treated with Chimeric Antigen Receptor T-cell(CART)Therapy[J]. Transplant Cell Ther, 2024, 30(9): 927. e1-927. e9. doi: 10.1016/j.jtct.2024.06.008
[25] Zhang M, Long X, Xiao Y, et al. Assessment and predictive ability of the absolute neutrophil count in peripheral blood for in vivo CAR T cells expansion and CRS[J]. J Immunother Cancer, 2023, 11(11): e007790. doi: 10.1136/jitc-2023-007790
[26] Pennisi M, Sanchez-Escamilla M, Flynn JR, et al. Modified EASIX predicts severe cytokine release syndrome and neurotoxicity after chimeric antigen receptor T cells[J]. Blood Adv, 2021, 5(17): 3397-3406. doi: 10.1182/bloodadvances.2020003885
[27] Zhao Y, Zhang X, Zhang M, et al. Modified EASIX scores predict severe CRS/ICANS in patients with acute myeloid leukemia following CLL1 CAR-T cell therapy[J]. Ann Hematol, 2024, 103(3): 969-980. doi: 10.1007/s00277-024-05617-y
[28] de Boer JW, Keijzer K, Pennings ERA, et al. Population-Based External Validation of the EASIX Scores to Predict CAR T-Cell-Related Toxicities[J]. Cancers(Basel), 2023, 15(22): 5443.
[29] Wang L, Lv Y, Zhou L, et al. Cytokine-based models for efficient differentiation between infection and cytokine release syndrome in patients with hematological malignancies[J]. Exp Hematol Oncol, 2024, 13(1): 28. doi: 10.1186/s40164-024-00495-6
[30] Wei Z, Zhao C, Zhang M, et al. PrCRS: a prediction model of severe CRS in CAR-T therapy based on transfer learning[J]. BMC Bioinformatics, 2024, 25(1): 197. doi: 10.1186/s12859-024-05804-8
[31] Jiang H, Liu L, Guo T, et al. Improving the safety of CAR-T cell therapy by controlling CRS-related coagulopathy[J]. Ann Hematol, 2019, 98(7): 1721-1732. doi: 10.1007/s00277-019-03685-z
[32] Galli E, Sorà F, Hohaus S, et al. Endothelial activation predicts disseminated intravascular coagulopathy, cytokine release syndrome and prognosis in patients treated with anti-CD19 CAR-T cells[J]. Br J Haematol, 2023, 201(1): 86-94. doi: 10.1111/bjh.18596
[33] Wang Y, Song Z, Geng Y, et al. The risk factors and early predictive model of hematotoxicity after CD19 chimeric antigen receptor T cell therapy[J]. Front Oncol, 2022, 12: 987965. doi: 10.3389/fonc.2022.987965
[34] Liu Y, Liang B, Liu Y, et al. Cytokine Release Syndrome Is an Independent Risk Factor Associated With Platelet Transfusion Refractoriness After CAR-T Therapy for Relapsed/Refractory Acute Lymphoblastic Leukemia[J]. Front Pharmacol, 2021, 12: 702152. doi: 10.3389/fphar.2021.702152
[35] Rejeski K, Perez A, Sesques P, et al. CAR-HEMATOTOX: a model for CAR T-cell-related hematologic toxicity in relapsed/refractory large B-cell lymphoma[J]. Blood, 2021, 138(24): 2499-2513. doi: 10.1182/blood.2020010543
[36] Frey NV, Shaw PA, Hexner EO, et al. Optimizing Chimeric Antigen Receptor T-Cell Therapy for Adults With Acute Lymphoblastic Leukemia[J]. J Clin Oncol, 2020, 38(5): 415-422. doi: 10.1200/JCO.19.01892
[37] Wen H, Huo G, Hou T, et al. Preclinical efficacy and safety evaluation of interleukin-6-knockdown CAR-T cells targeting at CD19[J]. Ann Transl Med, 2021, 9(23): 1713. doi: 10.21037/atm-21-3372
[38] Liu ZF, Chen LY, Wang J, et al. Successful treatment of acute B lymphoblastic leukemia relapse in the skin and testicle by anti-CD19 CAR-T with IL-6 knocking down: a case report[J]. Biomark Res, 2020, 8: 12. doi: 10.1186/s40364-020-00193-5
[39] Chen LY, Kang LQ, Zhou HX, et al. Successful application of anti-CD19 CAR-T therapy with IL-6 knocking down to patients with central nervous system B-cell acute lymphocytic leukemia[J]. Transl Oncol, 2020, 13(11): 100838. doi: 10.1016/j.tranon.2020.100838
[40] Gong WJ, Qiu Y, Li MH, et al. Investigation of the risk factors to predict cytokine release syndrome in relapsed or refractory B-cell acute lymphoblastic leukemia patients receiving IL-6 knocking down anti-CD19 chimeric antigen receptor T-cell therapy[J]. Front Immunol, 2022, 13: 922212. doi: 10.3389/fimmu.2022.922212
[41] Xue L, Yi Y, Xu Q, et al. Chimeric antigen receptor T cells self-neutralizing IL6 storm in patients with hematologic malignancy[J]. Cell Discov, 2021, 7(1): 84. doi: 10.1038/s41421-021-00299-6
[42] Lin MY, Nam E, Shih RM, et al. Self-regulating CAR-T cells modulate cytokine release syndrome in adoptive T-cell therapy[J]. J Exp Med, 2024, 221(6): e20221988. doi: 10.1084/jem.20221988
[43] Zhang P, Ying P, Li H, et al. A novel safer CD19CAR with shRNA interference of IFN-γ can reduce multiple cytokine levels without significantly compromising its killing efficacy[J]. Apoptosis, 2024, 29(3-4): 556-567. doi: 10.1007/s10495-023-01925-2
[44] Gong N, Han X, Xue L, et al. In situ PEGylation of CAR T cells alleviates cytokine release syndrome and neurotoxicity[J]. Nat Mater, 2023, 22(12): 1571-1580. doi: 10.1038/s41563-023-01646-6
[45] Li X, Gong N, Tian F, et al. Suppression of cytokine release syndrome during CAR-T-cell therapy via a subcutaneously injected interleukin-6-adsorbing hydrogel[J]. Nat Biomed Eng, 2023, 7(9): 1129-1141. doi: 10.1038/s41551-023-01084-4
[46] Schuster SJ, Svoboda J, Chong EA, et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas[J]. N Engl J Med, 2017, 377(26): 2545-2554. doi: 10.1056/NEJMoa1708566
[47] Costa BA, Flynn J, Nishimura N, et al. Prognostic impact of corticosteroid and tocilizumab use following chimeric antigen receptor T-cell therapy for multiple myeloma[J]. Blood Cancer J, 2024, 14(1): 84. doi: 10.1038/s41408-024-01048-0
[48] Wang X, Zhang B, Zhang Q, et al. Impact of tocilizumab on anti-CD19 chimeric antigen receptor T-cell therapy in B-cell acute lymphoblastic leukemia[J]. Cancer, 2024, 130(15): 2660-2669. doi: 10.1002/cncr.35316
[49] Perl M, Herfeld K, Harrer DC, et al. Tocilizumab administration in cytokine release syndrome is associated with hypofibrinogenemia after chimeric antigen receptor T-cell therapy for hematologic malignancies[J]. Haematologica, 2024, 109(9): 2969-2977.
[50] Poiret T, Vikberg S, Schoutrop E, et al. CAR T cells and T cells phenotype and function are impacted by glucocorticoid exposure with different magnitude[J]. J Transl Med, 2024, 22(1): 273. doi: 10.1186/s12967-024-05063-4
[51] Terao T, Kitamura W, Fujii N, et al. Negative Prognostic Impact of High-Dose or Long-Term Corticosteroid Use in Patients with Relapsed or Refractory B-Cell Lymphoma Who Received Tisagenlecleucel[J]. Transplant Cell Ther, 2023, 29(9): 573. e1-573. e8. doi: 10.1016/j.jtct.2023.06.018
[52] Pu Y, Zhao Y, Qi Y, et al. Multi-centers experience using therapeutic plasma exchange for corticosteroid/tocilizumab-refractory cytokine release syndrome following CAR-T therapy[J]. Int Immunopharmacol, 2024, 130: 111761. doi: 10.1016/j.intimp.2024.111761
[53] Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy-assessment and management of toxicities[J]. Nat Rev Clin Oncol, 2018, 15(1): 47-62. doi: 10.1038/nrclinonc.2017.148
[54] Thompson JA, Schneider BJ, Brahmer J, et al. NCCN Guidelines Insights: Management of Immunotherapy-Related Toxicities, Version 1.2020[J]. J Natl Compr Canc Netw, 2020, 18(3): 230-241. doi: 10.6004/jnccn.2020.0012
[55] Gutierrez C, Brown ART, Herr MM, et al. The chimeric antigen receptor-intensive care unit(CAR-ICU)initiative: Surveying intensive care unit practices in the management of CAR T-cell associated toxicities[J]. J Crit Care, 2020, 58: 58-64. doi: 10.1016/j.jcrc.2020.04.008
[56] Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas(TRANSCEND NHL 001): a multicentre seamless design study[J]. Lancet, 2020, 396(10254): 839-852. doi: 10.1016/S0140-6736(20)31366-0
[57] Yang Y, Zhang Y, Xing X, et al. IL-6 translation is a therapeutic target of human cytokine release syndrome[J]. J Exp Med, 2023, 220(11): e20230577. doi: 10.1084/jem.20230577
[58] Strati P, Ahmed S, Kebriaei P, et al. Clinical efficacy of anakinra to mitigate CAR T-cell therapy-associated toxicity in large B-cell lymphoma[J]. Blood Adv, 2020, 4(13): 3123-3127. doi: 10.1182/bloodadvances.2020002328
[59] Jain MD, Smith M, Shah NN. How I treat refractory CRS and ICANS after CAR T-cell therapy[J]. Blood, 2023, 141(20): 2430-2442.
[60] Diorio C, Vatsayan A, Talleur AC, et al. Anakinra utilization in refractory pediatric CAR T-cell associated toxicities[J]. Blood Adv, 2022, 6(11): 3398-3403. doi: 10.1182/bloodadvances.2022006983
[61] Zhang L, Wang S, Xu J, et al. Etanercept as a new therapeutic option for cytokine release syndrome following chimeric antigen receptor T cell therapy[J]. Exp Hematol Oncol, 2021, 10(1): 16. doi: 10.1186/s40164-021-00209-2
[62] 陈诗彧, 陈伟红, 万晓春, 等. 血液透析滤过处理CAR-T治疗后IL-6受体抑制剂治疗无效的3~4级细胞因子释放综合征3例[J]. 中华血液学杂志, 2022, 43(6): 494-498.
-

| 引用本文: | 李凌浩, 宫文洁, 吴德沛, 等. 聚焦血液肿瘤患者CAR-T治疗后细胞因子释放综合征[J]. 临床血液学杂志, 2025, 38(1): 77-83. doi: 10.13201/j.issn.1004-2806.2025.01.015 |
| Citation: | LI Linghao, GONG Wenjie, WU Depei, et al. CAR-T cell therapy associated cytokine release syndrome in hematologic tumors[J]. J Clin Hematol, 2025, 38(1): 77-83. doi: 10.13201/j.issn.1004-2806.2025.01.015 |
- Figure 1.
- Figure 2.



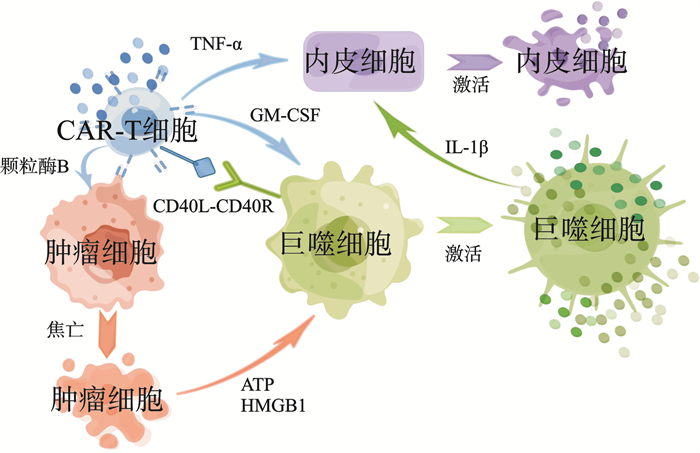
 下载:
下载: