Modified DAV in fit elderly patients with untreated de novo acute myeloid leukaemia: a single-centre retrospective analysis
-
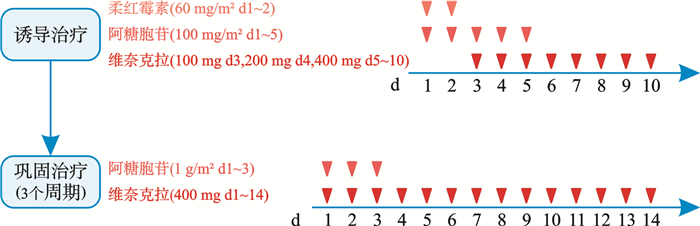
摘要: 目的 进一步探索维奈克拉联合“2+5”柔红霉素和阿糖胞苷(改良DAV方案)在适合强化疗的老年急性髓系白血病(AML)患者中的有效性和安全性。方法 对2021年3月—2023年7月接受改良DAV方案诱导化疗的25例老年AML患者进行了回顾性研究。分析总体缓解率(ORR)、复合完全缓解(CRc)率、总体生存期(OS)、无事件生存期(EFS)、缓解持续时间(DOR)以及不良事件等指标。结果 25例患者中,20例经过1个周期改良DAV诱导治疗后达到完全缓解(CR),3例经过1个周期改良DAV诱导治疗达到部分缓解(PR),经过2个周期改良DAV方案治疗后达到CR,2例未缓解。患者中位OS尚未达到,中位EFS为415 d。在诱导治疗过程中出现的不良事件包括3~4级中性粒细胞减少症(100.0%)、贫血(100.0%)、血小板减少症(100.0%)以及发热性中性粒细胞减少症(68.0%)。结论 改良DAV方案在老年适合强化疗的初发AML患者中具有良好的疗效和安全性。Abstract: Objective To explore the efficacy and safety of venetoclax in combination with '2+5' daunorubicin and cytarabine chemotherapy (modified DAV regimen) in fit elderly patients with acute myeloid leukemia (AML).Methods 25 elderly AML patients receiving modified DAV regimen induction chemotherapy from March 2021 to July 2023 were retrospectively analyzed. Overall response rate(ORR), composite complete response(CRc) rate, overall survival(OS), event free survival(EFS), duration of remission(DOR), and adverse events were analyzed.Results Among 25 patients, 20 patients achieved CR after one cycle of induction, 3 patients achieved PR after one cycle of induction and attained CR after a second modified DAV induction. 2 patients failed to achieve remission after induction. The median OS was not achieved and the median EFS was 415 days. The adverse events during the induction therapy were grade 3-4 neutropenia(100%), anemia(100%), thrombocytopenia(100%) and febrile neutropenia(68.0%).Conclusion Our study demonstrated promising response and safety of modified DAV therapy in elderly fit de novo AML patients.
-
Key words:
- elderly acute myeloid leukemia /
- modified DAV regimen /
- efficacy /
- safety
-
-
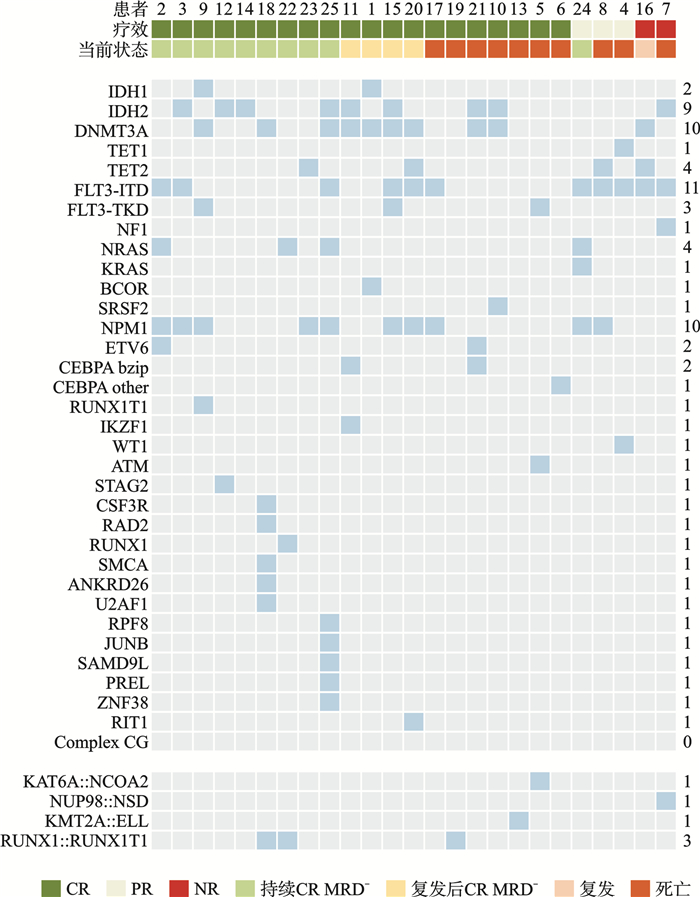
表 1 88个白血病相关突变基因列表
ANKRD26 ASXL1 ASXL2 BCOR BRAF CEBPA CSF3R DDX41 DHX15 DNMT3A EFL1 ETV6 EZH2 FLT3 GATA1 GATA2 IDH1 IDH2 IKZF2 IKZF3 KDM6A KIT KMT2A KRAS NF1 NPM1 NRAS PHF6 PTPN11 RAD21 RUNX1 SF3B1 SMC1A SMC3 SRP72 SRSF2 STAG2 STAT3 TET2 TP53 U2AF1 WT1 ZEB2 ZRSR2 ATM BCL11B BCORL1 CALR CBL CCND3 CDKN1B CTCF CUX1 DIS3 DNM2 ELANE EP300 FBXW7 GNAS IKZF1 JAK1 JAK2 JAK3 KDM5C KMT2C KMT2D MPL MSH6 MYC NOTCH1 NT5C2 PAX5 PDGFRA PDGFRB PPM1D PRPF8 PTEN RIT1 SBDS SETBP1 SETD2 SOS1 SPI1 SRCAP STAG1 SUZ12 TERC TERT 表 2 43个白血病相关融合基因列表
BCR∷ABL MLL∷AF4 TEL∷ABL NPM∷RARa MLL∷ENL NUP98∷HoxA11 CBFB∷MYH11 MLL∷AF6 TEL∷AML1 NUMA1∷RARa MLL∷ELL NUP98∷HoxA13 PML∷RARa MLL∷AF9 TEL∷JAK2 AML1∷ETO MLL∷AFX NUP98∷HoxC11 FIP1L1∷RARa MLL∷AF10 TEL∷PDGFRB AML1∷MDS1/EVI1 MLL∷SEPT6 NUP98∷HoxD13 PLZF∷RARa MLL∷AF17 FIP1L1∷PDGFRA AML1∷MTG16 SIL∷TAL1 NUP98∷PMX1 PRKAR1A∷RARa MLL∷AF1p ETV6∷PDGFRa NPM∷MLF1 E2A∷PBX1 TLS∷ERG STAT5b∷RARa MLL∷AF1q NUP98∷HoxA9 DEK∷CAN E2A∷HLF SET∷CAN 表 3 患者基线临床特征
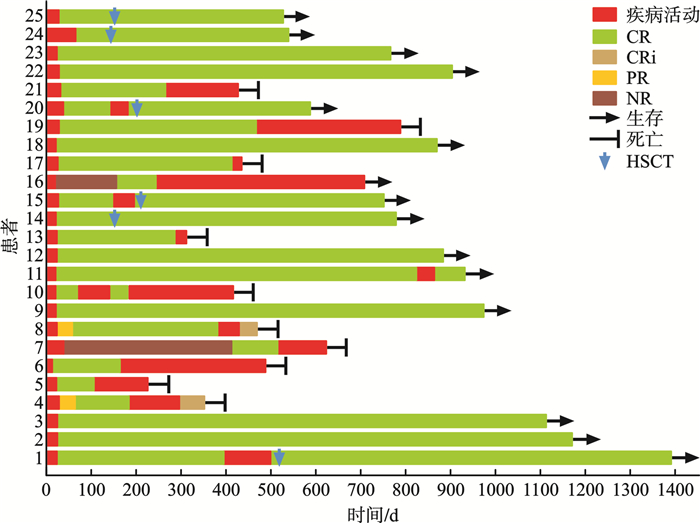
M(P25,P75),例(%) 临床特征 全部患者(n=25) 良好预后组(n=6) 中等预后组(n=14) 不良预后组(n=5) 年龄/岁 63(57,71) 64(60,68) 63(57,70) 62(61,71) 性别 男 9(36.0) 2(33.3) 6(42.9) 1(20.0) 女 16(64.0) 4(66.7) 8(57.1) 4(80.0) 体能状况评分/分 0(0,2) 0(0,2) 0.5(0,2) 0(0,1) 初诊WBC/(×109/L) 11.07(1.02,303.95) 13.17(1.38,105.43) 15.97(1.71,262.7) 2.34(1.02,303.95) 初诊骨髓原始细胞 < 30% 2(8.0) 0 1(7.1) 1(20.0) ≥30%且 < 50% 7(28.0) 2(33.3) 4(28.6) 1(20.0) ≥50% 16(64.0) 4(66.7) 9(64.3) 3(60.0) 表 4 患者疗效分析
例(%) 疗效 全部患者(n=25) 良好预后组(n=6) 中等预后组(n=14) 不良预后组(n=5) 总体反应率 23(92.0) 6(100.0) 12(85.7) 5(100.0) 复合完全缓解率 20(80.0) 6(100.0) 9(64.3) 5(100.0) CR率 20(80.0) 6(100.0) 9(64.3) 5(100.0) CRi率 0 0 0 0 PR率 3(12.0)1) 0 3(21.4) 0 NR 2(8.0) 0 2(14.3) 0 诱导治疗期间早期死亡 0 0 0 0 诱导治疗缓解患者中MRD阴性率 21(91.3) 5(83.3) 11(91.7) 5(100.0) DOR/d 467.0 未达到 467.0 326.0 OS/d 未达到 未达到 792.0 492.0 EFS/d 415.0 未达到 399.5 359.0 注:1)3例PR患者在二次诱导治疗后达到CR,在2个周期诱导治疗后CR率为92%。 表 5 患者安全性分析
例(%) 不良反应 诱导治疗
(n=25)第1周期巩固治疗
(n=22)第2周期巩固治疗
(n=20)第3周期巩固治疗
(n=17)全部
不良反应3~4级
不良反应全部
不良反应3~4级
不良反应全部
不良反应3~4级
不良反应全部
不良反应3~4级
不良反应血液学不良反应 血小板减少 25(100.0) 25(100.0) 22(100.0) 22(100.0) 20(100.0) 20(100.0) 17(100.0) 16(94.1) 中性粒细胞减少 25(100.0) 25(100.0) 22(100.0) 22(100.0) 20(100.0) 20(100.0) 17(100.0) 16(94.1) 贫血 25(100.0) 24(96.0) 22(100.0) 19(86.4) 20(100.0) 15(75.0) 17(100.0) 14(82.4) 非血液学不良反应 发热性中性粒细胞减少 17(68.0) 17(68.0) 5(22.7) 5(22.7) 5(25.0) 5(25.0) 3(17.6) 3(17.6) 恶心 8(32.0) 0 4(18.2) 0 3(15.0) 0 3(17.6) 0 呕吐 2(8.0) 0 2(9.1) 0 1(5.0) 0 2(11.8) 0 黏膜炎 5(20.0) 0 1(4.5) 0 2(10.0) 0 2(11.8) 0 腹泻 6(24.0) 1(4.0) 0 0 0 0 2(11.8) 0 便秘 7(28.0) 0 0 0 1(5.0) 0 0 0 皮疹 3(12.0) 0 1(4.5) 0 0 0 0 0 肺炎 8(32.0) 6(24.0) 8(36.4) 3(13.6) 3(15.0) 1(5.0) 3(17.6) 1(5.9) 脓毒血症 2(8.0) 2(8.0) 1(4.5) 1(4.5) 0 0 0 0 水肿 4(16.0) 0 0 0 1(5.0) 0 0 0 表 6 诱导治疗血细胞计数恢复时间(n=25)
M(P25,P75) 指标 恢复时间/da 中性粒细胞计数≥1×109/L,血小板计数≥50×109/L 18.00(16.75,22.00) 中性粒细胞计数≥0.5×109/L 17.00(15.00,19.25) 中性粒细胞计数≥1.0×109/L 18.00(16.00,22.00) 血小板计数≥20×109/L 14.00(12.50,16.50) 血小板计数≥50×109/L 18.00(15.00,19.00) 血小板计数≥100×109/L 19.50(17.25,22.00) 注:a自治疗第一天起。 -
[1] Lazarevic VL. Acute myeloid leukaemia in patients we judge as being older and/or unfit[J]. J Intern Med, 2021, 290(2): 279-293. doi: 10.1111/joim.13293
[2] Medeiros BC, Chan SM, Daver NG, et al. Optimizing survival outcomes with post-remission therapy in acute myeloid leukemia[J]. Am J Hematol, 2019, 94(7): 803-811. doi: 10.1002/ajh.25484
[3] DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia[J]. Blood, 2019, 133(1): 7-17. doi: 10.1182/blood-2018-08-868752
[4] DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia[J]. N Engl J Med, 2020, 383(7): 617-629. doi: 10.1056/NEJMoa2012971
[5] Wei AH, Montesinos P, Ivanov V, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial[J]. Blood, 2020, 135(24): 2137-2145. doi: 10.1182/blood.2020004856
[6] Wang H, Mao L, Yang M, et al. Venetoclax plus 3+7 daunorubicin and cytarabine chemotherapy as first-line treatment for adults with acute myeloid leukaemia: a multicentre, single-arm, phase 2 trial[J]. Lancet Haematol, 2022, 9(6): e415-e424. doi: 10.1016/S2352-3026(22)00106-5
[7] DiNardo CD, Lachowiez CA, Takahashi K, et al. Venetoclax Combined With FLAG-IDA Induction and Consolidation in Newly Diagnosed and Relapsed or Refractory Acute Myeloid Leukemia[J]. J Clin Oncol, 2021, 39(25): 2768-2778. doi: 10.1200/JCO.20.03736
[8] Zhou Z, Zhao X, Suo X, et al. Venetoclax Combined with Homoharringtonine, Cytarabine and Aclacinomycin(HAAV)As Induction Therapy in Newly Diagnosed Young Adult Acute Myeloid Leukemia[J]. Blood, 2023, 142(Supplement 1): 1517. doi: 10.1182/blood-2023-179275
[9] Wang H, Yao Y, Mao L, et al. Venetoclax plus '2 + 5' modified intensive chemotherapy with daunorubicin and cytarabine in fit elderly patients with untreated de novo acute myeloid leukaemia: a single-centre retrospective analysis[J]. Br J Haematol, 2023, 201(3): 568-572. doi: 10.1111/bjh.18709
[10] Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN[J]. Blood, 2022, 140(12): 1345-1377. doi: 10.1182/blood.2022016867
[11] Pollyea DA, Altman JK, Assi R, et al. Acute Myeloid Leukemia, Version 3.2023, NCCN Clinical Practice Guidelines in Oncology[J]. J Natl Compr Canc Netw, 2023, 21(5): 503-513. doi: 10.6004/jnccn.2023.0025
[12] National Cancer Institute. Common Terminology Criteria for Adverse Events(CTCAE), version 5.0.2017[S]. Published: November 27. National Cancer Institute, National Institutes of Health, United States Department of Health and Human Services.
[13] Chua CC, Roberts AW, Reynolds J, et al. Chemotherapy and Venetoclax in Elderly Acute Myeloid Leukemia Trial(CAVEAT): A Phase Ib Dose-Escalation Study of Venetoclax Combined With Modified Intensive Chemotherapy[J]. J Clin Oncol, 2020, 38(30): 3506-3517. doi: 10.1200/JCO.20.00572
[14] Chen XJ, Abuduaini D, Zhu HM, et al. Idarubicin Plus Azacitidine + Venetoclax As Induction Therapy Achieved High Remission in Elderly Fit-Acute Myeloid Leukemia Patients: Preliminary Results of a Phase Ⅱ Study[J]. Blood, 2023, 142(Supplement 1): 1527. doi: 10.1182/blood-2023-180049
[15] Muffly L, Pasquini MC, Martens M, et al. Increasing use of allogeneic hematopoietic cell transplantation in patients aged 70 years and older in the United States[J]. Blood, 2017, 130(9): 1156-1164. doi: 10.1182/blood-2017-03-772368
[16] Wei AH, Döhner H, Sayar H, et al. Long-term survival with oral azacitidine for patients with acute myeloid leukemia in first remission after chemotherapy: Updated results from the randomized, placebo-controlled, phase 3 QUAZAR AML-001 trial[J]. Am J Hematol, 2023, 98(4): E84-E87.
[17] Kapp-Schwoerer S, Weber D, Corbacioglu A, et al. Impact of gemtuzumab ozogamicin on MRD and relapse risk in patients with NPM1-mutated AML: results from the AMLSG 09-09 trial[J]. Blood, 2020, 136(26): 3041-3050. doi: 10.1182/blood.2020005998
[18] Reville PK, Kadia TM. Maintenance Therapy in AML[J]. Front Oncol, 2020, 10: 619085.
[19] Sorror ML, Sandmaier BM, Storer BE, et al. Long-term outcomes among older patients following nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation for advanced hematologic malignancies[J]. JAMA, 2011, 306(17): 1874-1883. doi: 10.1001/jama.2011.1558
[20] Versluis J, Hazenberg CL, Passweg JR, et al. Post-remission treatment with allogeneic stem cell transplantation in patients aged 60 years and older with acute myeloid leukaemia: a time-dependent analysis[J]. Lancet Haematol, 2015, 2(10): e427-36. doi: 10.1016/S2352-3026(15)00148-9
[21] Ivanov V, Yeh SP, Mayer J, et al. Design of the VIALE-M phase Ⅲ trial of venetoclax and oral azacitidine maintenance therapy in acute myeloid leukemia[J]. Future Oncol, 2022, 18(26): 2879-2889. doi: 10.2217/fon-2022-0450
[22] Konopleva M, Pollyea DA, Potluri J, et al. Efficacy and Biological Correlates of Response in a Phase Ⅱ Study of Venetoclax Monotherapy in Patients with Acute Myelogenous Leukemia[J]. Cancer Discov, 2016, 6(10): 1106-1117. doi: 10.1158/2159-8290.CD-16-0313
[23] Lagadinou ED, Sach A, Callahan K, et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells[J]. Cell Stem Cell, 2013, 12(3): 329-341. doi: 10.1016/j.stem.2012.12.013
[24] Pollyea DA, Stevens BM, Jones CL, et al. Venetoclax with azacitidine disrupts energy metabolism and targets leukemia stem cells in patients with acute myeloid leukemia[J]. Nat Med, 2018, 24(12): 1859-1866. doi: 10.1038/s41591-018-0233-1
[25] Kent A, Schwartz M, McMahon C, et al. Venetoclax is safe and tolerable as post-transplant maintenance therapy for AML patients at high risk for relapse[J]. Bone Marrow Transplant, 2023, 58(8): 849-854. doi: 10.1038/s41409-023-01987-5
-
计量
- 文章访问数: 215
- 施引文献: 0



 下载:
下载: