A single-center study of changes in the immune repertoire of cytomegalovirus-activated patients within six months after haploid allogeneic hematopoietic stem cell transplantation
-
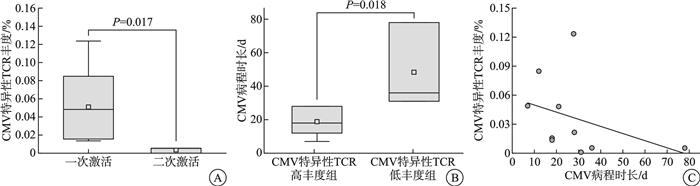
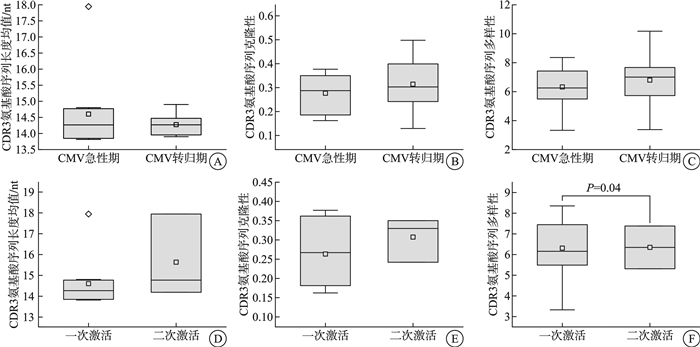
摘要: 目的 明确单倍体异基因造血干细胞移植(Haplo-HSCT)术后半年内巨细胞病毒(CMV)感染的发生情况,并探讨CMV激活及其转归过程中T细胞受体(TCR)组库变化的意义。方法 回顾性分析2022年1月—2024年1月在新疆医科大学第一附属医院血液中心行Haplo-HSCT的141例患者,评估其在移植后6个月内的CMV再激活情况,并监测CMV激活患者在感染急性期及转归期的TCR组库变化。结果 移植中心2年内行Haplo-HSCT患者共141例,移植后6个月内发生CMV再激活患者64例,累计再激活率45.39%,其中,57例(89.06%)出现CMV血症,7例(10.94%)发展为CMV病。清髓性预处理方案单倍体异基因造血干细胞移植(MAC Haplo-HSCT)38例与减低预处理剂量方案单倍体异基因造血干细胞移植(RIC Haplo-HSCT)26例进行组间比较,年龄差异有统计学意义(P < 0.001)。性别、CMV临床诊断、CMV发生时间、CMV持续时间、合并其他感染比较差异无统计学意义(P>0.05)。对10例首次CMV激活患者在急性期和转归期的TCR组库进行监测,结果显示3例患者出现二次激活。急性期与转归期外周血TCR指标(CDR3氨基酸序列长度均值、克隆性、多样性)之间差异无统计学意义(P>0.05)。对比一次激活患者与二次激活患者的TCR数据,发现一次激活患者在CMV感染急性期的TCR多样性差异有统计学意义(P < 0.05),而CDR3氨基酸序列长度均值及克隆性方面差异无统计学意义(P>0.05)。然而,一次激活患者的CMV特异性TCR丰度显著高于二次激活患者,差异有统计学意义(P < 0.05)。CMV特异性TCR丰度低于1%的患者病程显著长于高丰度组,且CMV特异性TCR丰度与CMV病程时长之间可能存在线性关系(P < 0.05)。结论 ① CMV再激活主要发生在Haplo-HSCT后3个月内,免疫功能低下是CMV激活的主要原因;②预处理方案对Haplo-HSCT患者发生CMV激活的影响不显著;③组库数据显示CMV二次激活患者体内可能缺乏足够的CMV特异性T细胞,导致对CMV感染的保护效力不足,病程延长;④CMV特异性TCR丰度可作为预测CMV二次激活的重要指标,并与CMV复发难治性高度相关。
-
关键词:
- 单倍体异基因造血干细胞移植 /
- 巨细胞病毒 /
- T细胞受体组库 /
- 复发难治
Abstract: Objective To determine the incidence of cytomegalovirus(CMV) infection within six months after haploid allogeneic hematopoietic stem cell transplantation(Haplo-HSCT) and to investigate the significance of changes in the T-cell receptor(TCR) repertoire during CMV activation and its resolution.Methods A retrospective analysis was conducted on 141 patients who underwent Haplo-HSCT at the Hematology Center of First Affiliated Hospital of Xinjiang Medical University between January 2022 and January 2024. The study assessed the incidence of CMV reactivation within six months post-transplantation and monitored the changes in the TCR repertoire during the infection and resolution phases in patients with CMV activation.Results Among the 141 patients who received Haplo-HSCT within the two-year period, 64(45.39%) experienced CMV reactivation within six months post-transplantation. Of these, 57 patients(89.06%) developed CMV viremia, and 7 patients(10.94%) progressed to CMV disease. A comparison between the myeloablative conditioning(MAC) Haplo-HSCT group(38 cases) and the reduced-intensity conditioning(RIC) Haplo-HSCT group(26 cases) showed a statistically significant difference in age(P < 0.001). No significant differences were observed between the two groups in terms of gender, clinical diagnosis of CMV, time to CMV onset, duration of CMV infection, or co-infections(P>0.05). TCR repertoire monitoring of 10 patients during their first episode of CMV activation revealed that 3 patients experienced a second episode of CMV reactivation. There was no significant difference in TCR indexes(mean length, clonality and diversity of CDR3 amino acid sequence) in peripheral blood between acute phase and resolution phase(P>0.05). It was found that there was a significant difference in TCR diversity between acute phase and resolution phase in peripheral blood TCR indices(P < 0.05), but no statistically significant differences were found in other peripheral blood TCR indices, including the mean CDR3 amino acid sequence length, and clonality(P>0.05). However, a comparison of TCR data between patients with a single CMV activation and those with a second activation indicated that the CMV-specific TCR abundance was significantly higher in patients with a single activation(P < 0.05). Patients with CMV-specific TCR abundance below 1% had a significantly longer disease course compared to those with higher TCR abundance, and there was a potential linear relationship between CMV-specific TCR abundance and the duration of CMV infection. (P < 0.05).Conclusion ① CMV reactivation primarily occurs within the first three months post-Haplo-HSCT, with immunosuppression being the primary cause of CMV activation. ②The conditioning regimen has a negligible impact on the incidence of CMV activation in Haplo-HSCT patients. ③The data suggest that patients with CMV reactivation may lack sufficient CMV-specific T cells, leading to inadequate protective immunity against CMV and a prolonged disease course. ④CMV-specific TCR abundance could serve as a predictive marker for CMV reactivation and is closely associated with the refractory nature of CMV relapse. -

-
表 1 不同预处理方案下Haplo-HSCT患者CMV发生情况
例(%) 项目 MAC RIC P 例数 38(59.38) 26(40.63) 年龄/岁 33.8
(9.0~52.0)49.3
(7.0~66.0)<0.001 性别 0.773 男 25(65.79) 18(69.23) 女 13(34.21) 8(30.77) CMV临床诊断 0.602 CMV血症 34(89.47) 23(88.46) CMV病 4(10.53) 3(11.54) CMV发生时间/d 50.1
(11.0~176.0)48.6
(29.0~63.0)0.797 CMV持续时间/d 20.1
(6.0~73.0)22.5
(4.0~58.0)0.443 合并其他感染 21(55.26) 13(50.00) 0.618 EB病毒感染 16(42.11) 9(34.62) 新冠病毒感染 3(7.89) 1(3.85) 真菌感染 2(5.26) 2(7.69) 细菌感染 0 1(3.85) 表 2 CMV激活急性期及转归期患者TCR变化及疾病转归
患者编号 感染急性期 转归期 CMV首次激活病程/d 二次激活 总克隆数 多样性 克隆性 总克隆数 多样性 克隆性 1 668 5.987 0.362 3 290 10.173 0.129 18 1 2 536 7.423 0.181 821 7.460 0.229 28 1 3 1 006 8.356 0.162 783 6.153 0.360 28 1 4 1 145 7.448 0.267 1 436 7.822 0.254 12 1 5 871 6.347 0.350 1 454 7.994 0.144 7 1 6 244 5.315 0.330 695 5.299 0.439 18 1 7 855 7.382 0.242 1 094 6.548 0.351 21 1 8 450 5.491 0.377 1 084 7.615 0.255 36 2 9 478 6.158 0.308 106 3.377 0.498 78 2 10 16 3.401 0.150 96 2.846 0.248 31 2 -
[1] 郑晓燕, 刘哲, 王晓宁, 等. 异基因造血干细胞移植后巨细胞病毒再激活的危险因素分析[J]. 临床血液学杂志, 2024, 37(5): 339-342, 348. doi: 10.13201/j.issn.1004-2806.2024.05.010
[2] Griffiths P, Reeves M. Pathogenesis of human cytomegalovirus in the immunocompromised host[J]. Nat Rev Microbiol, 2021, 19(12): 759-773. doi: 10.1038/s41579-021-00582-z
[3] Jerry Teng CL, Wang PN, Chen YC, et al. Cytomegalovirus management after allogeneic hematopoietic stem cell transplantation: A mini-review[J]. J Microbiol Immunol Infect, 2021, 54(3): 341-348. doi: 10.1016/j.jmii.2021.01.001
[4] Ljungman P, Schmitt M, Marty FM, et al. A Mortality Analysis of Letermovir Prophylaxis for Cytomegalovirus(CMV)in CMV-seropositive Recipients of Allogeneic Hematopoietic Cell Transplantation[J]. Clin Infect Dis, 2020, 70(8): 1525-1533. doi: 10.1093/cid/ciz490
[5] Kotton CN, Torre-Cisneros J, Yakoub-Agha I, et al. Slaying the "Troll of Transplantation"-new frontiers in cytomegalovirus management: A report from the CMV International Symposium 2023[J]. Transpl Infect Dis, 2024, 26(1): e14183. doi: 10.1111/tid.14183
[6] Li X, Liang H, Fan J. Prospects of Cytomegalovirus-Specific T-Cell Receptors in Clinical Diagnosis and Therapy[J]. Viruses, 2023, 15(6): 1334. doi: 10.3390/v15061334
[7] 中华医学会血液学分会干细胞应用学组. 异基因造血干细胞移植急性移植物抗宿主病诊断与治疗中国专家共识(2024年版)[J]. 中华血液学杂志, 2024, 45(6): 525-533.
[8] 王筱淇, 张曦. 《慢性移植物抗宿主病(cGVHD)诊断与治疗中国专家共识(2024年版)》解读[J]. 临床血液学杂志, 2024, 37(9): 597-601. doi: 10.13201/j.issn.1004-2806.2024.09.001
[9] 中华医学会血液学分会干细胞应用学组. 异基因造血干细胞移植患者巨细胞病毒感染管理中国专家共识(2022年版)[J]. 中华血液学杂志, 2022, 43(8): 617-623.
[10] Gao XN, Lin J, Wang LJ, et al. Risk factors and associations with clinical outcomes of cytomegalovirus reactivation after haploidentical versus matched-sibling unmanipulated PBSCT in patients with hematologic malignancies[J]. Ann Hematol, 2020, 99(8): 1883-1893. doi: 10.1007/s00277-020-04156-6
[11] 王乐玲, 莫文健, 张玉平, 等. 重型再生障碍性贫血异基因造血干细胞移植术后CMV感染的临床分析[J]. 中国实验血液学杂志, 2021, 29(3): 944-950.
[12] Shah K, Al-Haidari A, Sun J, et al. T cell receptor(TCR)signaling in health and disease[J]. Signal Transduct Target Ther, 2021, 6(1): 412. doi: 10.1038/s41392-021-00823-w
[13] Ruan Y, Luo T, Liu Q, et al. Features of cytomegalovirus infection and evaluation of cytomegalovirus-specific T cells therapy in children's patients following allogeneic hematopoietic stem cell transplantation: A retrospective single-center study[J]. Front Cell Infect Microbiol, 2022, 12: 1027341. doi: 10.3389/fcimb.2022.1027341
[14] Lin CH, Su YJ, Hsu CY, et al. Haploidentical allogeneic hematopoietic stem cell transplantation increases the risk of cytomegalovirus infection in adult patients with acute leukemia[J]. Transpl Infect Dis, 2019, 21(4): e13096. doi: 10.1111/tid.13096
[15] Ljungman P, de la Camara R, Robin C, et al. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia(ECIL 7)[J]. Lancet Infect Dis, 2019, 19(8): e260-e272. doi: 10.1016/S1473-3099(19)30107-0
[16] Jakharia N, Howard D, Riedel DJ. CMV Infection in Hematopoietic Stem Cell Transplantation: Prevention and Treatment Strategies[J]. Curr Treat Options Infect Dis, 2021, 13(3): 123-140. doi: 10.1007/s40506-021-00253-w
[17] Robin C, Thiebaut A, Alain S, et al. Letermovir for Secondary Prophylaxis of Cytomegalovirus Infection and Disease after Allogeneic Hematopoietic Cell Transplantation: Results from the French Compassionate Program[J]. Biol Blood Marrow Transplant, 2020, 26(5): 978-984. doi: 10.1016/j.bbmt.2020.01.027
[18] Serio B, Giudice V, Guariglia R, et al. Prophylactic letermovir decreases cytomegalovirus reactivation after stem cell transplantation: a single-center real-world evidence study[J]. Infez Med, 2021, 29(1): 102-113.
[19] Eberhardt KA, Jung V, Knops E, et al. CMV-IgG pre-allogeneic hematopoietic stem cell transplantation and the risk for CMV reactivation and mortality[J]. Bone Marrow Transplant, 2023, 58(6): 639-646. doi: 10.1038/s41409-023-01944-2
[20] Yang D, Yao Y, Sun Y, et al. Refractory cytomegalovirus infections in Chinese patients receiving allogeneic hematopoietic cell transplantation: a review of the literature[J]. Front Immunol, 2023, 14: 1287456. doi: 10.3389/fimmu.2023.1287456
[21] 熊艺颖, 刘林, 陈建斌, 等. 异基因造血干细胞移植术后巨细胞病毒感染的临床研究[J]. 中国实验血液学杂志, 2023, 31(2): 513-521.
[22] Szmit Z, Frączkiewicz J, Salamonowicz-Bodzioch M, et al. The Impact of High CMV Viral Load and Refractory CMV Infection on Pediatric HSCT Recipients with Underlying Non-Malignant Disorder[J]. J Clin Med, 2022, 11(17): 5187. doi: 10.3390/jcm11175187
[23] Chu ND, Bi HS, Emerson RO, et al. Longitudinal immunosequencing in healthy people reveals persistent T cell receptors rich in highly public receptors[J]. BMC Immunol, 2019, 20(1): 19. doi: 10.1186/s12865-019-0300-5
[24] Joshi K, de Massy MR, Ismail M, et al. Spatial heterogeneity of the T cell receptor repertoire reflects the mutational landscape in lung cancer[J]. Nat Med, 2019, 25(10): 1549-1559. doi: 10.1038/s41591-019-0592-2
[25] Schultheiß C, Paschold L, Simnica D, et al. Next-Generation Sequencing of T and B Cell Receptor Repertoires from COVID-19 Patients Showed Signatures Associated with Severity of Disease[J]. Immunity, 2020, 53(2): 442-455. e4. doi: 10.1016/j.immuni.2020.06.024
[26] Sanz-Pamplona R, Melas M, Maoz A, et al. Lymphocytic infiltration in stage Ⅱ microsatellite stable colorectal tumors: A retrospective prognosis biomarker analysis[J]. PLoS Med, 2020, 17(9): e1003292. doi: 10.1371/journal.pmed.1003292
[27] Schäfer A, Calderin Sollet Z, Hervé MP, et al. NK-and T-cell repertoire is established early after allogeneic HSCT and is imprinted by CMV reactivation[J]. Blood Adv, 2024, 8(21): 5612-5624. doi: 10.1182/bloodadvances.2024013117
[28] Nakasone H, Kusuda M, Terasako-Saito K, et al. Features of repertoire diversity and gene expression in human cytotoxic T cells following allogeneic hematopoietic cell transplantation[J]. Commun Biol, 2021, 4(1): 1177. doi: 10.1038/s42003-021-02709-7
[29] Kuzich JA, Kankanige Y, Guinto J, et al. T cell receptor beta locus sequencing early post-allogeneic stem cell transplant identifies patients at risk of initial and recurrent cytomegalovirus infection[J]. Bone Marrow Transplant, 2021, 56(10): 2582-2590. doi: 10.1038/s41409-021-01354-2
[30] Seo S, Smith C, Fraser C, et al. Adoptive T-cell therapy for pediatric cytomegalovirus-associated retinitis[J]. Blood Adv, 2019, 3(11): 1774-1777. doi: 10.1182/bloodadvances.2019000121
[31] Liu G, Chen H, Cao X, et al. Efficacy of pp65-specific TCR-T cell therapy in treating cytomegalovirus infection after hematopoietic stem cell transplantation[J]. Am J Hematol, 2022, 97(11): 1453-1463. doi: 10.1002/ajh.26708
-

计量
- 文章访问数: 277
- 施引文献: 0



 下载:
下载: