Measurable residual disease guided therapy for patients with acute lymphoblastic leukemia
-
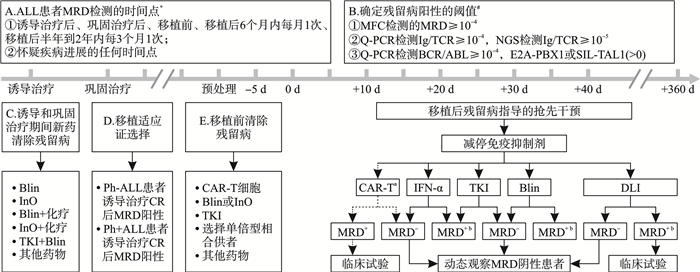
摘要: 微小残留病(MRD)已被常规用于急性淋巴细胞白血病(ALL)患者的疗效评估和复发预警。文章从初诊患者诱导和巩固治疗方案的选择、异基因造血干细胞移植适应证确定、移植供者选择、预处理方案以及移植后复发干预等方面讨论了MRD指导的ALL分层治疗。未来的研究应关注残留病检测新技术以及残留病清除新方法的建立。Abstract: Measurable residual disease(MRD) has been used routinely for curative effect evaluation and relapse forecast for patients with acute lymphoblastic leukemia(ALL). The article discussed MRD guided precision treatment for ALL in view of induction therapy and consolidation therapy selction, allogeneic hematolopoietic stem cell transplantation(allo-HSCT) indication determination, transplant donor selection, conditioning regimen and relapse prevention after allo-HSCT. In future, we should focus on the establishment of new methods for MRD evaluation and novel strategies for MRD eradication.
-

-
[1] Malard F, Mohty M. Acute lymphoblastic leukaemia[J]. Lancet, 2020, 395(10230): 1146-1162. doi: 10.1016/S0140-6736(19)33018-1
[2] Gökbuget N, Boissel N, Chiaretti S, et al. Management of ALL in adults: 2024 ELN recommendations from a European expert panel[J]. Blood, 2024, 143(19): 1903-1930. doi: 10.1182/blood.2023023568
[3] Gong X, Fang Q, Gu R, et al. A pediatric-inspired regimen for adolescent and adult patients with Philadelphia chromosome-negative acute lymphoblastic leukemia: a prospective study from China[J]. Haematologica, 2024, 109(10): 3146-3156. doi: 10.3324/haematol.2023.284228
[4] Jabbour EJ, Short NJ, Jain N, et al. Blinatumomab is associated with favorable outcomes in patients with B-cell lineage acute lymphoblastic leukemia and positive measurable residual disease at a threshold of 10-4 and higher[J]. Am J Hematol, 2022, 97(9): 1135-1141. doi: 10.1002/ajh.26634
[5] Jabbour E, Haddad FG, Short NJ, et al. Phase 2 study of inotuzumab ozogamicin for measurable residual disease in acute lymphoblastic leukemia in remission[J]. Blood, 2024, 143(5): 417-421. doi: 10.1182/blood.2023022330
[6] Vogiatzi F, Winterberg D, Lenk L, et al. Daratumumab eradicates minimal residual disease in a preclinical model of pediatric T-cell acute lymphoblastic leukemia[J]. Blood, 2019, 134(8): 713-716. doi: 10.1182/blood.2019000904
[7] Topp MS, Kufer P, Gökbuget N, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival[J]. J Clin Oncol, 2011, 29(18): 2493-2498. doi: 10.1200/JCO.2010.32.7270
[8] Kim R, Chalandon Y, Rousselot P, et al. Significance of Measurable Residual Disease in Adult Philadelphia Chromosome-Positive ALL: A GRAAPH-2014 Study[J]. J Clin Oncol, 2024, 42(26): 3140-3150. doi: 10.1200/JCO.24.00108
[9] Gökbuget N, Boissel N, Chiaretti S, et al. Diagnosis, prognostic factors, and assessment of ALL in adults: 2024 ELN recommendations from a European expert panel[J]. Blood, 2024, 143(19): 1891-1902. doi: 10.1182/blood.2023020794
[10] Pui CH, Pei D, Coustan-Smith E, et al. Clinical utility of sequential minimal residual disease measurements in the context of risk-based therapy in childhood acute lymphoblastic leukaemia: a prospective study[J]. Lancet Oncol, 2015, 16(4): 465-474. doi: 10.1016/S1470-2045(15)70082-3
[11] Zhang Y, Wang S, Zhang J, et al. Elucidating minimal residual disease of paediatric B-cell acute lymphoblastic leukaemia by single-cell analysis[J]. Nat Cell Biol, 2022, 24(2): 242-252. doi: 10.1038/s41556-021-00814-7
[12] Saygin C, Cannova J, Stock W, et al. Measurable residual disease in acute lymphoblastic leukemia: methods and clinical context in adult patients[J]. Haematologica, 2022, 107(12): 2783-2793. doi: 10.3324/haematol.2022.280638
[13] Gökbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia[J]. Blood, 2018, 131(14): 1522-1531. doi: 10.1182/blood-2017-08-798322
[14] Yan CH, Liu DH, Liu KY, et al. Risk stratification-directed donor lymphocyte infusion could reduce relapse of standard-risk acute leukemia patients after allogeneic hematopoietic stem cell transplantation[J]. Blood, 2012, 119(14): 3256-3262. doi: 10.1182/blood-2011-09-380386
[15] Wang Y, Chang YJ, Chen J, et al. Consensus on the monitoring, treatment, and prevention of leukaemia relapse after allogeneic haematopoietic stem cell transplantation in China: 2024 update[J]. Cancer Lett, 2024, 605: 217264. doi: 10.1016/j.canlet.2024.217264
[16] Sun YQ, Li SQ, Zhao XS, et al. Measurable residual disease of acute lymphoblastic leukemia in allograft settings: how to evaluate and intervene[J]. Expert Rev Anticancer Ther, 2020, 20(6): 453-464. doi: 10.1080/14737140.2020.1766973
[17] Brüggemann M, Raff T, Flohr T, et al. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia[J]. Blood, 2006, 107(3): 1116-1123. doi: 10.1182/blood-2005-07-2708
[18] Berry DA, Zhou S, Higley H, et al. Association of Minimal Residual Disease With Clinical Outcome in Pediatric and Adult Acute Lymphoblastic Leukemia: A Meta-analysis[J]. JAMA Oncol, 2017, 3(7): e170580. doi: 10.1001/jamaoncol.2017.0580
[19] Gupta S, Rau RE, Kairalla JA, et al. Blinatumomab in Standard-Risk B-Cell Acute Lymphoblastic Leukemia In Children[J]. N Engl J Med, 2025, 392(9): 875-891. doi: 10.1056/NEJMoa2411680
[20] Jabbour E, Richard-Carpentier G, Sasaki Y, et al. Hyper-CVAD regimen in combination with ofatumumab as frontline therapy for adults with Philadelphia chromosome-negative B-cell acute lymphoblastic leukaemia: a single-arm, phase 2 trial[J]. Lancet Haematol, 2020, 7(7): e523-e533. doi: 10.1016/S2352-3026(20)30144-7
[21] Jabbour E, Short NJ, Jain N, et al. Ponatinib and blinatumomab for Philadelphia chromosome-positive acute lymphoblastic leukaemia: a US, single-centre, single-arm, phase 2 trial[J]. Lancet Haematol, 2023, 10(1): e24-e34. doi: 10.1016/S2352-3026(22)00319-2
[22] Jabbour E, Short NJ, Jain N, et al. Hyper-CVAD and sequential blinatumomab for newly diagnosed Philadelphia chromosome-negative B-cell acute lymphocytic leukaemia: a single-arm, single-centre, phase 2 trial[J]. Lancet Haematol, 2022, 9(12): e878-e885. doi: 10.1016/S2352-3026(22)00285-X
[23] Jabbour E, Short NJ, Senapati J, et al. Mini-hyper-CVD plus inotuzumab ozogamicin, with or without blinatumomab, in the subgroup of older patients with newly diagnosed Philadelphia chromosome-negative B-cell acute lymphocytic leukaemia: long-term results of an open-label phase 2 trial[J]. Lancet Haematol, 2023, 10(6): e433-e444. doi: 10.1016/S2352-3026(23)00073-X
[24] Kantarjian H, Short NJ, Haddad FG, et al. Results of the Simultaneous Combination of Ponatinib and Blinatumomab in Philadelphia Chromosome-Positive ALL[J]. J Clin Oncol, 2024, 42(36): 4246-4251. doi: 10.1200/JCO.24.00272
[25] Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia[J]. N Engl J Med, 2017, 376(9): 836-847. doi: 10.1056/NEJMoa1609783
[26] Litzow MR, Sun Z, Mattison RJ, et al. Blinatumomab for MRD-Negative Acute Lymphoblastic Leukemia in Adults[J]. N Engl J Med, 2024, 391(4): 320-333. doi: 10.1056/NEJMoa2312948
[27] Roddie C, Sandhu KS, Tholouli E, et al. Obecabtagene Autoleucel in Adults with B-Cell Acute Lymphoblastic Leukemia[J]. N Engl J Med, 2024, 391(23): 2219-2230. doi: 10.1056/NEJMoa2406526
[28] Stelljes M, Raffel S, Alakel N, et al. Inotuzumab Ozogamicin as Induction Therapy for Patients Older Than 55 Years With Philadelphia Chromosome-Negative B-Precursor ALL[J]. J Clin Oncol, 2024, 42(3): 273-282. doi: 10.1200/JCO.23.00546
[29] Logan AC. Measurable residual disease in acute lymphoblastic leukemia: How low is low enough?[J]. Best Pract Res Clin Haematol, 2022, 35(4): 101407. doi: 10.1016/j.beha.2022.101407
[30] Giebel S, Marks DI, Boissel N, et al. Hematopoietic stem cell transplantation for adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first remission: a position statement of the European Working Group for Adult Acute Lymphoblastic Leukemia(EWALL)and the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation(EBMT)[J]. Bone Marrow Transplant, 2019, 54(6): 798-809. doi: 10.1038/s41409-018-0373-4
[31] Short NJ, Jabbour E, Albitar M, et al. Recommendations for the assessment and management of measurable residual disease in adults with acute lymphoblastic leukemia: A consensus of North American experts[J]. Am J Hematol, 2019, 94(2): 257-265. doi: 10.1002/ajh.25338
[32] Snowden JA, Sánchez-Ortega I, Corbacioglu S, et al. Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022[J]. Bone Marrow Transplant, 2022, 57(8): 1217-1239. doi: 10.1038/s41409-022-01691-w
[33] Ribera JM, Oriol A, Morgades M, et al. Treatment of high-risk Philadelphia chromosome-negative acute lymphoblastic leukemia in adolescents and adults according to early cytologic response and minimal residual disease after consolidation assessed by flow cytometry: final results of the PETHEMA ALL-AR-03 trial[J]. J Clin Oncol, 2014, 32(15): 1595-1604. doi: 10.1200/JCO.2013.52.2425
[34] Dholaria B, Savani BN, Labopin M, et al. Clinical applications of donor lymphocyte infusion from an HLA-haploidentical donor: consensus recommendations from the Acute Leukemia Working Party of the EBMT[J]. Haematologica, 2020, 105(1): 47-58. doi: 10.3324/haematol.2019.219790
[35] Duell J, Dittrich M, Bedke T, et al. Frequency of regulatory T cells determines the outcome of the T-cell-engaging antibody blinatumomab in patients with B-precursor ALL[J]. Leukemia, 2017, 31(10): 2181-2190. doi: 10.1038/leu.2017.41
[36] Köhnke T, Krupka C, Tischer J, et al. Increase of PD-L1 expressing B-precursor ALL cells in a patient resistant to the CD19/CD3-bispecific T cell engager antibody blinatumomab[J]. J Hematol Oncol, 2015, 8: 111. doi: 10.1186/s13045-015-0213-6
[37] Zhao Y, Aldoss I, Qu C, et al. Tumor-intrinsic and-extrinsic determinants of response to blinatumomab in adults with B-ALL[J]. Blood, 2021, 137(4): 471-484. doi: 10.1182/blood.2020006287
[38] Wintering A, Ishiyama K, Tamaki S, et al. CD22low/Bcl-2high expression identifies poor response to inotuzumab ozogamicin in relapsed/refractory acute lymphoblastic leukemia[J]. Blood Adv, 2023, 7(2): 251-255. doi: 10.1182/bloodadvances.2021006810
[39] Zhao Y, Short NJ, Kantarjian HM, et al. Genomic determinants of response and resistance to inotuzumab ozogamicin in B-cell ALL[J]. Blood, 2024, 144(1): 61-73. doi: 10.1182/blood.2024023930
[40] Zheng S, Gillespie E, Naqvi AS, et al. Modulation of CD22 Protein Expression in Childhood Leukemia by Pervasive Splicing Aberrations: Implications for CD22-Directed Immunotherapies[J]. Blood Cancer Discov, 2022, 3(2): 103-115. doi: 10.1158/2643-3230.BCD-21-0087
[41] Escherich CS, Moriyama T, Li Z, et al. DNTT-mediated DNA damage response drives inotuzumab ozogamicin resistance in B-cell acute lymphoblastic leukemia[J]. Blood, 2025, 145(11): 1182-1194. doi: 10.1182/blood.2024026085
[42] Bhatla T, Hogan LE, Teachey DT, et al. Daratumumab in pediatric relapsed/refractory acute lymphoblastic leukemia or lymphoblastic lymphoma: the DELPHINUS study[J]. Blood, 2024, 144(21): 2237-2247. doi: 10.1182/blood.2024024493
-

计量
- 文章访问数: 72
- 施引文献: 0



 下载:
下载: