A retrospective study of polatuzumab vedotin-based combination regimens in the treatment of newly diagnosed diffuse large B-Cell lymphoma
-
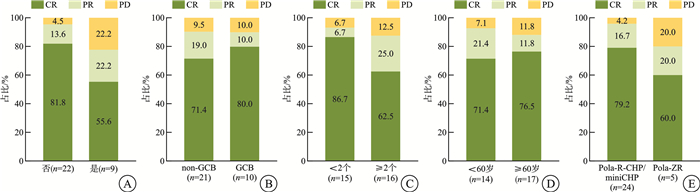
摘要: 目的 探讨基于维泊妥珠单抗(Pola)的联合治疗方案在初治弥漫性大B细胞淋巴瘤(DLBCL)患者中的疗效与安全性。方法 对2023年4月—2025年2月在华中科技大学同济医学院附属协和医院行Pola联合治疗的31例初治DLBCL患者进行回顾性病例资料整理与分析。结果 共纳入31例患者,中位年龄62(18~81)岁,其中非生发中心B细胞(non-GCB)型21例(67.7%),双表达淋巴瘤(DEL)9例(29.0%),结外受累27例(87.1%),国际预后指数评分3~5分者18例(58.1%)。使用Pola-R-CHP(Pola联合利妥昔单抗、环磷酰胺、多柔比星及泼尼松)方案治疗者22例,Pola-miniCHP方案2例,Pola-ZR(Pola联合泽布替尼和利妥昔单抗)方案5例,其余2例分别为Pola-BR(Pola联合苯达莫司汀和利妥昔单抗)及Pola-R-GDP(Pola联合利妥昔单抗、吉西他滨、地塞米松和顺铂)方案。疗效评估显示,客观缓解率(ORR)为90.3%,完全缓解(CR)率为74.2%,疾病进展(PD)率为9.7%。DEL患者的ORR为77.8%,CR率为55.6%;non-GCB患者的ORR为90.5%,CR率为71.4%。老年患者(≥60岁)ORR为92.9%,CR率为71.4%。Pola-R-CHP/miniCHP组ORR为95.8%,CR率为79.2%;Pola-ZR组ORR为80.0%,CR率为60.0%。预期1年总体生存(OS)率为92.4%,无进展生存(PFS)率为84.9%。不同分组在ORR、CR率、OS率和PFS率方面差异无统计学意义。治疗过程中,最常见的血液学相关不良事件包括贫血(轻度58.1%、重度12.9%),中性粒细胞减少(轻度16.1%、重度19.4%)及白细胞减少(轻度25.8%、重度19.4%);非血液系统不良事件包括肺部感染(29.0%)、食欲减退(12.9%)、恶心与呕吐(各9.7%)。结论 在真实世界研究背景下,基于Pola的联合方案在初治DLBCL患者中显示出较高的缓解率与较好耐受性。在DEL、non-GCB、伴结外侵犯及老年等高风险人群中亦表现出潜在的治疗前景,有待进一步研究以明确其临床获益。
-
关键词:
- 弥漫性大B细胞淋巴瘤 /
- 维泊妥珠单抗 /
- 初治 /
- 疗效 /
- 安全性
Abstract: Objective To investigate the efficacy and safety of polatuzumab vedotin(Pola) -based combination regimens in patients with newly diagnosed diffuse large B-cell lymphoma(DLBCL).Methods A retrospective analysis was conducted on the clinical data of 31 patients with newly diagnosed DLBCL who received Pola-based combination regimens at Union Hospital, Tongji Medical College, Huazhong University of Science and Technology from April 2023 to February 2025.Results A total of 31 patients were included, with a median age of 62(range 18-81) years. Among them, 21 patients(67.7%) had the non-germinal center B-cell-like(non-GCB) subtype, 9 patients(29.0%) had double-expressor lymphoma(DEL), and 27 patients(87.1%) presented with extranodal involvement. Eighteen patients(58.1%) had an IPI score of 3-5. The treatment regimens included Pola-R-CHP(n=22), Pola-miniCHP(n=2), Pola-ZR(n=5), Pola-BR(n=1) and Pola-R-GDP(n=1). The objective response rate(ORR) was 90.3%, with a complete response(CR) rate of 74.2% and a progressive disease(PD) rate of 9.7%. DEL patients had an ORR of 77.8% and a CR rate of 55.6%; non-GCB patients had an ORR of 90.5% and a CR rate of 71.4%. Elderly patients(≥60 years) had an ORR of 92.9% and a CR rate of 71.4%. The ORR and CR rate were 95.8% and 79.2% in the Pola-R-CHP/miniCHP group, and 80.0% and 60.0% in the Pola-ZR group, respectively. The estimated 1-year overall survival(OS) and progression-free survival(PFS) rates were 92.4% and 84.9%, respectively. There were no statistically significant differences in ORR, CR, OS or PFS among the subgroups. The most common treatment-related adverse events were anemia(grade 1-2: 58.1%, grade 3-4: 12.9%), neutropenia(grade 1-2: 16.1%, grade 3-4: 19.4%), and leukopenia(grade 1-2: 25.8%, grade 3-4: 19.4%); the most frequent non-hematological toxicities included pulmonary infection(29.0%), decreased appetite(12.9%), nausea and vomiting(each 9.7%).Conclusion In real-world settings, Pola-based combination regimens demonstrated favorable remission rates and manageable safety profiles in newly diagnosed DLBCL patients, with potential clinical value in high-risk subgroups such as DEL, non-GCB subtype, extranodal involvement, and elderly patients. Further investigation is warranted to confirm these benefits.-
Key words:
- diffuse large B-cell lymphoma /
- polatuzumab vedotin /
- newly diagnosed /
- efficacy /
- safety
-

-
表 1 31例初治DLBCL患者基本情况
中位数(范围),例(%) 特征 数值 中位年龄/岁 62(18~81) 性别 男 13(41.9) 女 18(58.1) ECOG评分/分 0~1 19(61.3) ≥ 2 12(38.7) B症状 无 20(64.5) 有 11(35.5) 诊断时乳酸脱氢酶 正常 11(35.5) 升高 20(64.5) Lugano分期/期 Ⅰ~Ⅱ 7(22.6) Ⅲ~Ⅳ 24(77.4) IPI评分/分 0~2 13(41.9) 3~5 18(58.1) 细胞起源 GCB 10(32.3) non-GCB 21(67.7) DEL 否 22(71.0) 是 9(29.0) 骨髓受累 无 30(96.8) 有 1(3.2) 结外累及 无 4(12.9) 有 27(87.1) 结外病灶数(限结外受累患者)/个 <2 11(40.7) ≥ 2 16(59.3) 中位随访时间/d 180(42~657) 中位PFS时间/d 158(42~625) 注:以下3种情况中出现任何1种即有B症状:①不明原因发热>38℃,连续3 d以上,排除感染的原因;②夜间盗汗(可浸透衣物);③体重于诊断前半年内下降>10%。 表 2 患者治疗相关不良反应情况
例(%) 不良反应类别 1~2级 3~4级 血液学不良反应 贫血 18(58.1) 4(12.9) 血小板减少 3(9.7) 5(16.1) 白细胞减少 8(25.8) 6(19.4) 中性粒细胞减少 5(16.1) 6(19.4) 发热性中性粒细胞减少 4(12.9) 非血液学不良反应 肺部感染 9(29.0) 过敏 1(3.2) 心律失常 2(6.5) 肝功能不良 2(6.5) 食欲减退 4(12.9) 恶心 3(9.7) 呕吐 3(9.7) -
[1] Geng H, Jia S, Zhang Y, et al. Efficacy and safety of zanubrutinib plus R-CHOP in treatment of non-GCB DLBCL with extranodal involvement[J]. Front Immunol, 2023, 14: 1219167. doi: 10.3389/fimmu.2023.1219167
[2] Sehn LH, Salles G. Diffuse Large B-Cell Lymphoma[J]. N Engl J Med, 2021, 384(9): 842-858. doi: 10.1056/NEJMra2027612
[3] Poletto S, Novo M, Paruzzo L, et al. Treatment strategies for patients with diffuse large B-cell lymphoma[J]. Cancer Treat Rev, 2022, 110: 102443. doi: 10.1016/j.ctrv.2022.102443
[4] 张钰奇, 武盈盈, 张奥, 等. 基于奥布替尼的联合方案治疗28例初治弥漫性大B细胞淋巴瘤的疗效与安全性分析[J]. 临床血液学杂志, 2024, 37(9): 631-636. doi: 10.13201/j.issn.1004-2806.2024.09.007
[5] McMillan AK, Phillips EH, Kirkwood AA, et al. Favourable outcomes for high-risk diffuse large B-cell lymphoma(IPI 3-5) treated with front-line R-CODOX-M/R-IVAC chemotherapy: results of a phase 2 UK NCRI trial[J]. Ann Oncol, 2020, 31(9): 1251-1259. doi: 10.1016/j.annonc.2020.05.016
[6] Zelenetz AD, Gordon LI, Abramson JS, et al. NCCN Guidelines® Insights: B-Cell Lymphomas, Version 6.2023[J]. J Natl Compr Canc Netw, 2023, 21(11): 1118-1131. doi: 10.6004/jnccn.2023.0057
[7] 中国临床肿瘤学会指南工作委员会. 中国临床肿瘤学会(CSCO)淋巴瘤诊疗指南2023[M]. 北京: 人民卫生出版社, 2023: 28.
[8] Tilly H, Morschhauser F, Sehn LH, et al. Polatuzumab vedotin in previously untreated diffuse large B-cell lymphoma[J]. N Engl J Med, 2022, 386(4): 351-363. doi: 10.1056/NEJMoa2115304
[9] Deng R, Gibiansky L, Lu T, et al. Population pharmacokinetics and exposure-response analyses of polatuzumab vedotin in patients with previously untreated DLBCL from the POLARIX study[J]. CPT Pharmacometrics Syst Pharmacol, 2024, 13(6): 1055-1066. doi: 10.1002/psp4.13141
[10] Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification[J]. J Clin Oncol, 2014, 32(27): 3059-3068. doi: 10.1200/JCO.2013.54.8800
[11] Freites-Martinez A, Santana N, Arias-Santiago S, et al. Using the common terminology criteria for adverse events(CTCAE-Version 5.0) to evaluate the severity of adverse events of anticancer therapies[J]. Actas Dermosifiliogr(Engl Ed), 2021, 112(1): 90-92. doi: 10.1016/j.ad.2019.05.009
[12] Shanthi D, Vinotha S. A comprehensive review of Polatuzumab vedotin: Mechanisms, clinical applications, and future prospects[J]. Natl Board Exam J Med Sci, 2024, 2(3): 256-263.
[13] Dornan D, Bennett F, Chen Y, et al. Therapeutic potential of an anti-CD79b antibody-drug conjugate, anti-CD79b-vc-MMAE, for the treatment of non-Hodgkin lymphoma[J]. Blood, 2009, 114(13): 2721-2729. doi: 10.1182/blood-2009-02-205500
[14] Song Y, Tilly H, Rai S, et al. Polatuzumab vedotin in previously untreated DLBCL: an Asia subpopulation analysis from the phase 3 POLARIX trial[J]. Blood, 2023, 141(16): 1971-1981. doi: 10.1182/blood.2022017734
[15] Mehta A, Verma A, Gupta G, et al. Double hit and double expresser diffuse large B cell lymphoma subtypes: discrete subtypes and major predictors of overall survival[J]. Indian J Hematol Blood Transfus, 2020, 36(4): 627-634. doi: 10.1007/s12288-019-01248-w
[16] Zhao P, Zhao S, Huang C, et al. Efficacy and safety of Polatuzumab vedotin plus rituximab, cyclophosphamide, doxorubicin and prednisone for previously untreated diffuse large B-cell lymphoma: a real-world, multi-center, retrospective cohort study[J]. Hematol Oncol, 2025, 43(1): e70017. doi: 10.1002/hon.70017
[17] Russler-Germain DA, Cliff ERS, Bartlett NL. Cell-of-origin effect of polatuzumab vedotin in diffuse large B-cell lymphoma: no ordinary subgroup analysis[J]. Blood, 2023, 142(25): 2216-2219. doi: 10.1182/blood.2023022048
[18] Gupta V, Singh V, Bajwa R, et al. Site-specific survival of extra nodal diffuse large B-cell lymphoma and comparison with gastrointestinal diffuse large B-cell lymphoma[J]. J Hematol, 2022, 11(2): 45-54. doi: 10.14740/jh984
[19] Ayers EC, Smith SM. Diffuse large B-cell lymphoma in the older and frail patient[J]. Cancers(Basel), 2025, 17(5): 885.
[20] Jardin F, Tilly H. Chemotherapy-free treatment in unfit patients aged 75 years and older with DLBCL: toward a new paradigm?[J]. Lancet Healthy Longev, 2022, 3(7): e453-e454. doi: 10.1016/S2666-7568(22)00150-7
-

计量
- 文章访问数: 70
- 施引文献: 0



 下载:
下载: