The efficacy of venetoclax combined with azacytidine in the treatment of recurrence after allogeneic hematopoietic stem cell transplantation
-
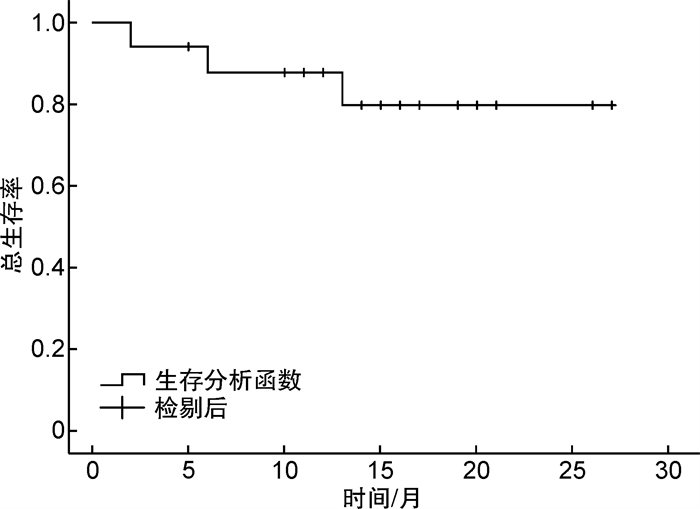
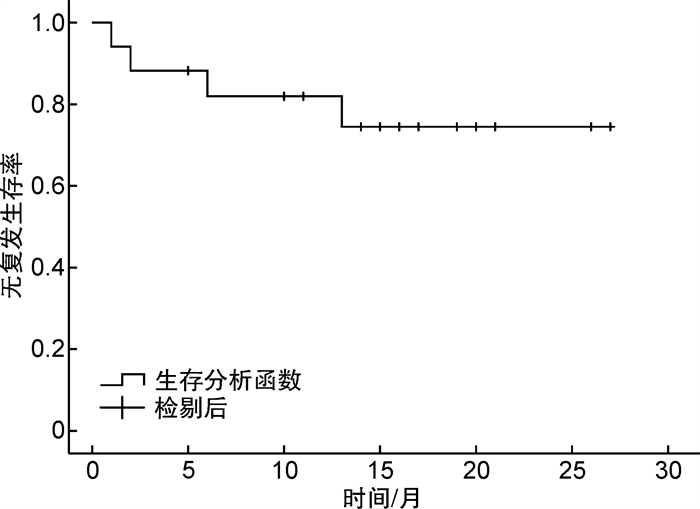
摘要: 目的 观察维奈克拉联合阿扎胞苷抢先治疗急性髓系白血病/骨髓增生异常综合征异基因造血干细胞移植后复发的疗效。方法 回顾性分析我科2020年1月1日—2022年10月31日共17例异基因造血干细胞移植后出现微小残留病(minimal residual disease,MRD)阳性的急性髓系白血病/骨髓增生异常综合征患者,使用维奈克拉联合阿扎胞苷进行抢先治疗。结果 中位随访时间18(4~28)个月,所有患者使用维奈克拉联合阿扎胞苷1~5个疗程。17例患者中15例评价为有效,总有效率为88.2%。治疗有效的15例患者中,7例1个疗程后MRD阴性,5例2个疗程后MRD转阴,3例3个疗程后MRD转阴。2例评价为疗效不佳。随访期间共死亡3例:2例因疾病进展死亡;1例因合并感染死亡。抢先治疗后中位无复发生存时间为15(1~27)个月,中位总生存时间为15(2~27)个月。抢先治疗后预期2年无复发生存率为74.5%,预期2年总生存率为79.9%。结论 维奈克拉联合阿扎胞苷抢先治疗可使造血干细胞移植后分子生物学复发的患者再次达微小残留病阴性,延长移植后无复发生存率,提高造血干细胞移植的疗效,且不良反应小,耐受性良好。
-
关键词:
- 异基因造血干细胞移植 /
- 微小残留病 /
- 阿扎胞苷 /
- 维奈克拉
Abstract: Objective To evaluate the clinical efficacy of venetoclax combined with azacytidine in the treatment of early recurrence after allogeneic hematopoietic stem cell transplantation.Methods A total of 17 acute myeloid leukemia/myelodysplastic syndrome patients with minimal residual disease(MRD) positive after allogeneic hematopoietic stem cell transplantation from Jan 1st, 2020 to Oct 31st, 2022 were analyzed retrospectively and pre-treated with venetoclax combined with azacytidine.Results The median time of follow-up after pre-treatment was 18 months(ranging 4 to 28). All of the 17 patients received venetoclax combined with azacytidine for 1-5 cycles, and 15 of 17 cases were evaluated as effective, with a total effective rate of 88.2%. The other 2 cases were evaluated as having poor curative effect. Among the 15 patients who received effective treatment, 7 cases had negative MRD after 1 course of treatment, 5 cases had negative MRD after 2 courses of treatment, and 3 cases had negative MRD after 3 courses of treatment. During the follow-up period, there were 3 cases that died: 2 cases died of progression of the disease and 1 case died of infection. The median relapse-free survival time was 15 months(1-27 months) and the median overall survival time was 15 months(2-27 months) after preemptive treatment. The expected 2-year relapse-free survival and 2-year overall survival after preemptive treatment were 74.5% and 79.9%.Conclusion Pre-treatment with venetoclax combined with azacytidine is effective for patients who had MRD after allogeneic stem cell transplantation. -

-
表 1 17例allo-HSCT后MRD阳性患者的临床特征
序号 性别 年龄/岁 移植前诊断 HLA配型 预处理方案 移植至MRD阳性时间/月 抢先治疗后随访时间/月 1 男 24 AML,CR1 同胞全合 CAB 4 28 2 男 31 AML,CR1 同胞全合 FAB 5 28 3 女 37 AML,CR1,MRD+ 单倍体 CAB 5 25 4 男 20 AML,CR2 同胞全合 CAB 10 4 5 男 57 MDS,NR 单倍体 改良Bu/Cy 4 22 6 男 40 AML,CR1 同胞全合 FAB 12 22 7 男 23 AML,CR2,MRD+ 单倍体 CAB 5 21 8 男 25 AML,CR1 单倍体 CAB 14 18 9 女 22 AML,CR1 同胞全合 CAB 7 18 10 女 26 AML,CR1 单倍体 FAB 4 14 11 男 24 AML,CR2,MRD+ 单倍体 CAB 10 19 12 男 19 AML,CR1 单倍体 CAB 6 4 13 女 43 MDS,CR1,MRD+ 同胞7/12 CAB 11 17 14 女 38 AML,CR1 同胞全合 FAB 3 13 15 女 27 AML,NR 单倍体 CAB 2 9 16 男 21 AML,NR 单倍体 CAB 1 15 17 男 49 AML,CR1,MRD+ 单倍体 CAB 7 8 表 2 17例患者抢先治疗的疗效
序号 疗程 治疗反应 GVHD 骨髓抑制 治疗后缓解持续时间/d 转归 疗效评价 1 1 MRD- 无 1~2度 27 MRD- 有效 2 2 MRD- 无 3~4度 26 MRD- 有效 3 4 MRD- 无 1~2度 21 MRD- 有效 4 3 形态学复发 无 1~2度 1 疾病进展死亡 无效 5 1 MRD- 无 1~2度 20 MRD- 有效 6 3 MRD- 无 1~2度 19 MRD- 有效 7 4 MRD- 无 1~2度 17 MRD- 有效 8 2 MRD- 皮肤Ⅱ度aGVHD 1~2度 16 MRD- 有效 9 3 MRD- 无 1~2度 15 MRD- 有效 10 2 MRD- 无 3~4度 13 新冠肺炎死亡 有效 11 3 MRD- 无 3~4度 15 MRD- 有效 12 2 MRD- 无 无 2 供者淋巴细胞输注后MRD- 无效 13 3 MRD- 无 1~2度 14 MRD- 有效 14 2 MRD- 无 3~4度 11 MRD- 有效 15 3 MRD- 无 1~2度 6 MRD-后6个月,形态学复发死亡 有效 16 5 MRD+ 无 1~2度 10 MRD- 有效 17 2 MRD- 无 无 5 MRD- 有效 -
[1] Schuler E, Boughoufala S, Rautenberg C, et al. Relapse patterns and treatment strategies in patients receiving allogeneic hematopoietic stem cell transplantation for myeloid malignancies[J]. Ann Hematol, 2019, 98(5): 1225-1235. doi: 10.1007/s00277-019-03670-6
[2] Daver N, Garcia MG, Basu S, et al. Efficacy, safety, and biomarkers of response to azacitidine and nivolumab in relapsed/refractory acute myeloid leukemia: a nonrandomized, open-label Phase Ⅱ Study[J]. Cancer Discov, 2019, 9(3): 370-383. doi: 10.1158/2159-8290.CD-18-0774
[3] Oran B. Is there a role for therapy after transplant?[J]. Best Pract Res Clin Haematol, 2015, 28(2-3): 124-132. doi: 10.1016/j.beha.2015.10.009
[4] Platzbecker U, Middeke JM, Sockel K, et al. Measurable residual disease-guided treatment with azacitidine to prevent haematological relapse in patients with myelodysplastic syndrome and acute myeloid leukaemia(RELAZA2): an open-label, multicentre, phase 2 trial[J]. Lancet Oncol, 2018, 19(12): 1668-1679. doi: 10.1016/S1470-2045(18)30580-1
[5] 肖方, 刘强, 郭欢绪, 等. 地西他滨治疗异基因造血干细胞移植后早期复发的疗效观察[J]. 临床血液学杂志, 2020, 33(1): 29-32. doi: 10.13201/j.issn.1004-2806.2020.01.007 https://lcxy.whuhzzs.com/article/doi/10.13201/j.issn.1004-2806.2020.01.007
[6] Kent A, Pollyea DA, Winters A, et al. Venetoclax is safe and tolerable as post-transplant maintenance therapy for AML patients at high risk for relapse[J]. Bone Marrow Transplant, 2023 Apr 25. doi: 10.1038/s41409-023-01987-5.Epubaheadofprint.
[7] Gabriel SS, Bon N, Chen J, et al. Distinctive Expression of Bcl-2 Factors in Regulatory T Cells Determines a Pharmacological Target to Induce Immunological Tolerance[J]. Front Immunol, 2016, 7: 73.
[8] Nguyen LXT, Troadec E, Kalvala A, et al. The Bcl-2 inhibitor venetoclax inhibits Nrf2 antioxidant pathway activation induced by hypomethylating agents in AML[J]. J Cell Physiol, 2019, 234(8): 14040-14049. doi: 10.1002/jcp.28091
[9] Tsai JJ, Velardi E, Shono Y, et al. Nrf2 regulates CD4(+)T cell-induced acute graft-versus-host disease in mice[J]. Blood, 2018, 132(26): 2763-2774. doi: 10.1182/blood-2017-10-812941
[10] 杨莉莉, 王淑君, 胡婉贞, 等. 阿扎胞苷联合维奈克拉治疗老年急性髓系白血病患者临床疗效分析[J]. 临床血液学杂志, 2022, 35(7): 512-516. doi: 10.13201/j.issn.1004-2806.2022.07.012 https://lcxy.whuhzzs.com/article/doi/10.13201/j.issn.1004-2806.2022.07.012
[11] Muller-TC, Schlenk RF. A new option for remission induction in acute myeloid leukaemia[J]. Lancet Oncol, 2018, 19(2): 156-157. doi: 10.1016/S1470-2045(18)30012-3
[12] Pollyea DA, Stevens BM, Jones CL, et al. Venetoclax with azacitidine disrupts energy metabolism and targets leukemia stem cells in patients with acute myeloid leukemia[J]. Nat Med, 2018, 24(12): 1859-1866. doi: 10.1038/s41591-018-0233-1
[13] Wei Y, Xiong X, Li X, et al. Low-dose decitabine plus venetoclax is safe and effective as post-transplant maintenance therapy for high-risk acute myeloid leukemia and myelodysplastic syndrome[J]. Cancer Sci, 2021, 112(9): 3636-3644. doi: 10.1111/cas.15048
[14] Esther S, Eva-Maria WD, Salem A, et al. Treatment of myeloid malignancies relapsing after allogeneic hematopoietic stem cell transplantation with venetoclax and hypomethylating agents-a retrospective multicenter analysis on behalf of the German Cooperative Transplant Study Group[J]. Ann Hematol, 2021, 100(4): 959-968. doi: 10.1007/s00277-020-04321-x
[15] Zhao P, Ni M, Ma D, et al. Venetoclax plus azacitidine and donor lymphocyte infusion in treating acute myeloid leukemia patients who relapse after allogeneic hematopoietic stem cell transplantation[J]. Ann Hematol, 2022, 101(1): 119-130. doi: 10.1007/s00277-021-04674-x
[16] 李可芯, 赵薇薇, 刘瑶维, 等. 维奈克拉血药浓度监测在治疗急性髓系白血病中的价值及联合阿扎胞苷治疗急性髓系白血病的疗效与安全性分析[J]. 临床血液学杂志, 2022, 35(11): 812-816. https://lcxy.whuhzzs.com/article/doi/10.13201/j.issn.1004-2806.2022.11.012
[17] Schroeder T, Rautenberg C, Haas R, et al. Hypomethylating agents for treatment and prevention of relapse after allogeneic blood stem cell transplantation[J]. Int J Hematol, 2018, 107(2): 138-150. doi: 10.1007/s12185-017-2364-4
[18] Platzbecker U, Middeke JM, Sockel K, et al. Measurable residual disease-guided treatment with azacitidine to prevent haematological relapse in patients with myelodysplastic syndrome and acute myeloid leukaemia(RELAZA2): an openlabel, multicentre, phase 2 trial[J]. Lancet Oncol, 2018, 19(12): 1668-1679. doi: 10.1016/S1470-2045(18)30580-1
[19] Guillaume T, Malard F, Magro L, et al. Prospective phase Ⅱ study of prophylactic low-dose azacitidine and donor lymphocyte infusions following allogeneic hematopoietic stem cell transplantation for high-risk acute myeloid leukemia and myelodysplastic syndrome[J]. Bone Marrow Transplant, 2019, 54(11): 1815-1826. doi: 10.1038/s41409-019-0536-y
-




 下载:
下载: